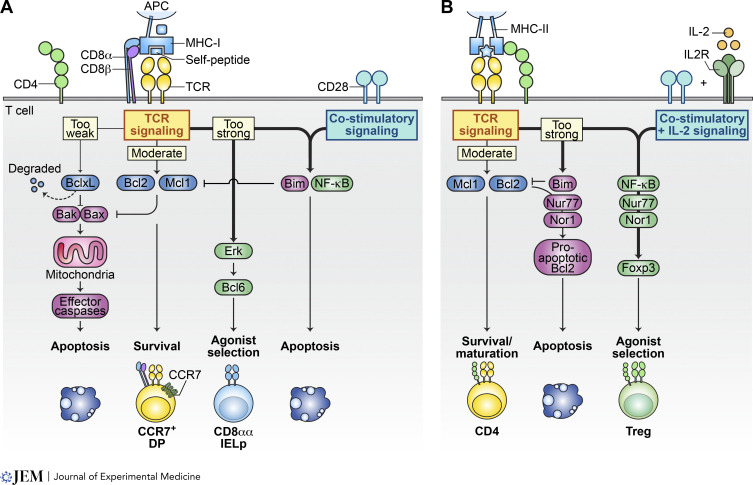
Figure 3.
Differential signaling in agonist selection versus negative selection. (A and B) This illustration delineates the differential signaling pathways that guide thymocytes undergoing selection thymocytes toward apoptosis or toward agonist selection into CD8αα IEL precursors (IELp; A) or Treg cells (B). (A) MHC-I–restricted DP thymocytes receiving moderate TCR signaling can upregulate pro-survival proteins Bcl2 and Mcl1, which take over the survival signaling role from the degrading BclxL. Thymocytes that fail to sufficiently engage TCR signaling to upregulate Bcl2 or Mcl1 will succumb to apoptosis due to BclxL degradation. Conversely, excessively strong TCR signaling leads to thymocyte apoptosis through a mechanism involving NF-κB and pro-apoptotic Bim. Intriguingly, in the absence of NF-κB involvement, MHC-I–restricted thymocytes receiving strong signals may differentiate into CD8αα IEL precursors. This process is facilitated by strong Erk signaling and the upregulation of transcription factor Bcl6, marking an alternative agonist-selection pathway exclusive to MHC-I–restricted thymocytes in the cortex. (B) Conversely, MHC-II–restricted thymocytes have an alternative agonist selection pathway leading to Treg cell development in the medulla. Here, moderate TCR signaling is again pivotal for upregulating pro-survival Bcl2 and Mcl1, aiding in the maturation of CD4 T cells. However, overly strong signaling triggers apoptosis. Unlike for CD8αα IEL precursors, co-stimulatory signaling and IL-2 signaling in this context do not induce apoptosis but rather support development of Treg cells.