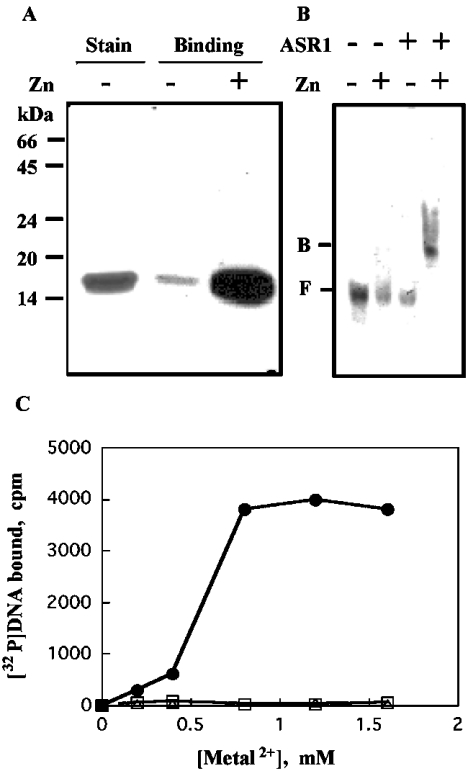
Figure 4. ASR1 possesses a zinc-dependent DNA-binding activity.
ASR1 was expressed in E. coli and purified to homogeneity. Purified ASR1 was assayed for DNA-binding using radiolabelled DNA, as described in the Experimental section. (A) For South-Western-blot analysis, membranes containing ASR1 were incubated with the radiolabelled 50-bp DNA probe and exposed to X-ray film (see the Experimental section). Stain, Coomassie Blue staining of purified ASR1. (B) Mobility shift assays: binding mixtures (containing the radiolabelled 50-bp DNA probe) were loaded on to 5% (w/v) polyacrylamide gels exposed to X-ray film (see the Experimental section). F, free; B, bound. (C) For filtration through nitrocellulose, reaction mixtures containing in addition radiolabelled 227-bp pBluescript polylinker DNA probe and various bivalent metal ions were incubated and filtered through 0.22 μm nitrocellulose membranes using a slot-blot manifold (see the Experimental section). Membrane-retained radioactivity was determined for ZnCl2 (•), MgCl2 (▵) and CaCl2 (□).