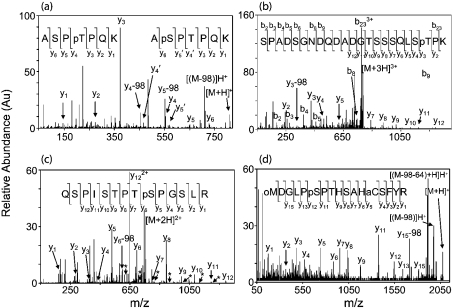
Figure 3. Direct sequencing of the phosphorylation sites using tandem MS/MS.
(a) ESI–MS/MS spectrum of the fragmentation of the singly charged peptide at m/z 808.35 from sample i (Figure 2a). Two separate, and complete, series of y ions describe the phosphopeptides DCX286–292, where either Ser-287 or Thr-289 is phosphorylated. Ions marked with a prime are exclusive to the sequence containing phospho-Ser-287. The rest of the y ions are overlapping. Ions demonstrating neutral loss of phosphoric acid (−98 Da) are also shown (indicated by [(M-98)H]+). (b) ESI–MS/MS spectrum of the fragmentation of the triply charged m/z 782.32 from sample iii (Figure 2a). The spectrum matches the sequence for DCX306–328, where Thr-326 is phosphorylated and Asn-317 has been deamidated. (c) ESI–MS/MS spectrum of the fragmentation of the doubly charged m/z 754.41 from sample v (Figure 2a). The sequence derived from this spectrum matches DCX331–344, where Ser-339 is phosphorylated. There is also a second very low abundance y ion series in the spectrum (marked with *). This series best describes DCX331–334, where Ser-332 is phosphorylated. (d) MALDI-MS/MS spectrum of the singly charged parent ion at m/z 2072.86 from sample vi (Figure 2a). The spectrum reveals the sequence of DCX23–39, where Met-23 is oxidized (oM), Asn-24 has been deamidated, Cys-35 has been modified by acrylamide (aC) and Ser-28 is phosphorylated. The neutral loss of phosphoric acid and the abundant further neutral loss of methane sulphenic acid (CH3SOH, −64 Da) are also shown.