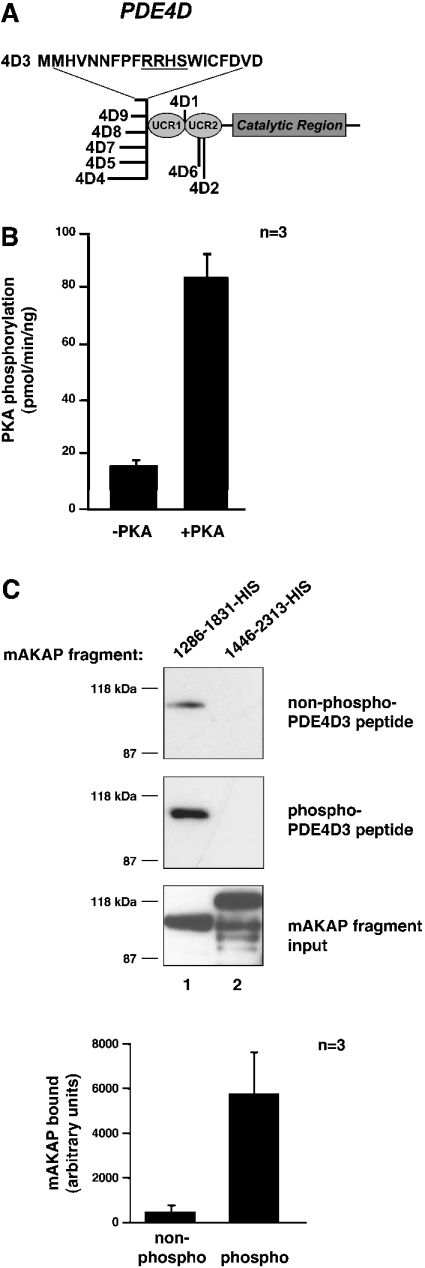
Figure 2. mAKAP 1286–1831 binds PKA-phosphorylated PDE4D3 peptide better than non-phosphorylated peptide in vitro.
(A) The structure of the PDE4D family of PDEs. The conserved catalytic core and upstream conserved regions (UCRs) are indicated. The locations of divergent sequences for each isoform are indicated, and the 20 unique amino acid residues of PDE4D3 are denoted by the one-letter amino acid code. The PKA-phosphorylation consensus site is underlined. (B) Phosphorylation of PDE4D3 peptide by PKA. PDE4D3 peptide was incubated in PKA kinase buffer containing 100 μM ATP, 5 μM [γ-32P]ATP and 0.3 mM cAMP with or without the PKA catalytic subunit for 15 min at 30 °C. The reaction mixture was spotted on to p81 phosphocellulose paper, washed five times in 75 mM phosphoric acid and once in 95% ethanol. Filters were air-dried and were counted by liquid-scintillation counting. Results are averages of three independent experiments. (C) Pull-down assay of mAKAP fragments with PDE4D3 peptide. Biotin–PDE4D3 peptide was incubated in PKA kinase buffer containing 100 μM ATP and 0.3 mM cAMP with or without the PKA catalytic subunit for 2 h at 30 °C. Neutravidin beads were added to collect the peptide and then rocked for 1 h at room temperature. The precipitates were washed three times and resuspended in 500 μl of HSE buffer containing 5 μM recombinant mAKAP fragments. Reactions were incubated overnight at 4 °C with rocking. Precipitates were washed three times with HSE buffer, and bound proteins were subjected to SDS/7% PAGE followed by immunoblotting with anti-His antibody. The top blot tests co-precipitation of mAKAP fragments with nonphosphorylated PDE4D3 peptide. The middle blot tests co-precipitation of mAKAP fragments with phosphorylated PDE4D3 peptide. The bottom blot shows that equal amounts of mAKAP fragments (lane 1, mAKAP 1286–1831–His; lane 2, mAKAP 1446–2313–His) were used in the assay. The 1286–1831–His bands from the top and middle blots were quantified using NIH Image software. The amount of mAKAP fragment bound to non-phosphorylated (non-phospho) and PKA-phosphorylated (phospho) peptides was graphed in arbitrary units. Results are means±S.E.M. for three independent experiments.