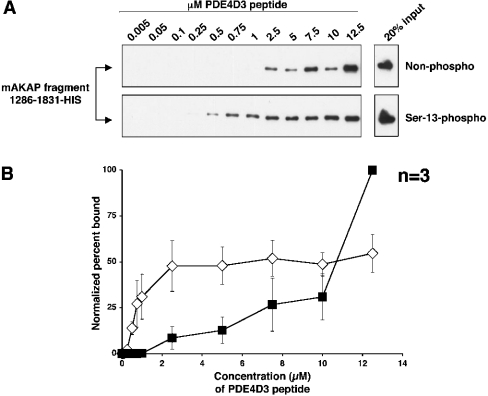
Figure 3. The mAKAP 1286–1831 fragment binds to lower concentrations of Ser-13-phosphorylated PDE4D3 peptide than non-phosphorylated peptide in vitro.
(A) Biotin–PDE4D3 peptides that were either non-phosphorylated (upper panel) or phosphorylated on Ser-13 (lower panel) were mixed at various concentrations (indicated in μM) with a fixed amount of mAKAP fragment 1286–1831–His. Biotin–PDE4D3 peptides were collected by Neutravidin beads, resolved by SDS/7% PAGE and subjected to Western analysis using anti-His antibody to detect the mAKAP fragment. The upper and lower right-hand panels represent 20% of the mAKAP fragment added to the samples. The blots shown are representative of three independent experiments. (B) Bands from (A) were quantified using NIH Image for each experiment, and the percentage of mAKAP 1286–1831–His bound to peptide for each concentration point was determined (see the Experimental section). To create the graph, the normalized percentage of mAKAP 1286–1831–His bound to biotin–Ser-13-phosphorylated PDE4D3 peptide (⋄) or biotin–non-phosphorylated PDE4D3 peptide (▪) was plotted against peptide concentration. Results are the means±S.E.M. for three independent experiments.