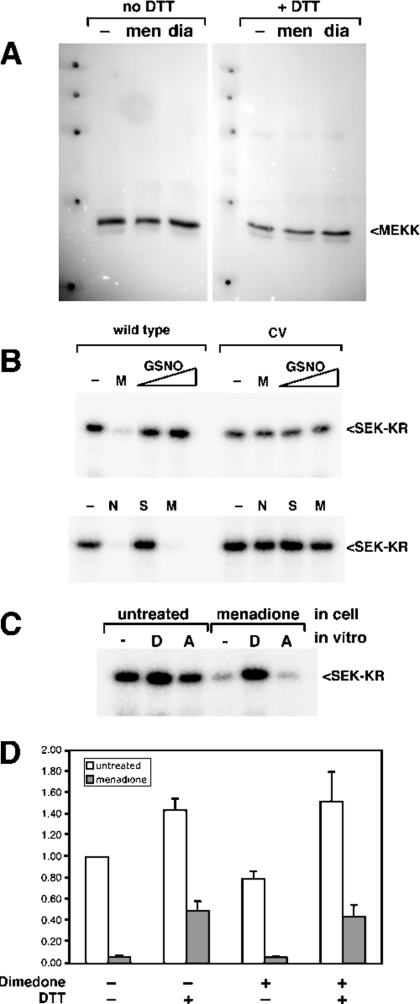
Figure 6. MEKK1 inhibition by oxidants does not involve inter- or intra-molecular disulphide-bond formation, NO modification, or oxidation of cysteine to sulphenic acid.
(A) Cells expressing the catalytic domain fragment of MEKK1 were left untreated (‘–’) or treated with menadione (‘men’; 250 μM) or diamide (‘dia’; 1 mM). The cells were lysed and samples prepared in Laemmli sample buffer without DTT supplemented with 10 mM NEM. Samples were divided into two fractions and one was adjusted to 75 mM DTT. The samples were subjected to SDS/10%-PAGE, transferred to PVDF membrane and blotted with anti-MEKK1 antibody. Oxidant-inhibited MEKK1 did not exhibit altered electrophoretic mobility under non-reducing conditions. (B) Cells expressing wild-type or C1238V (CV) mutant Δ1172 (upper panel) or N-terminally truncated MEKK1 Δ765 (lower pannel) were treated with menadione (M, 250 μM), GSNO (0.2 mM or 0.5 mM), NEM (N, 1 mM) or SNAP (S, 100 μM) as indicated. MEKK1 was purified and activity measured by in vitro kinase assay. MEKK1 activity was unaffected by either of the NO donors. (C) The catalytic domain of MEKK1 was isolated from either untreated cells or cells exposed to 250 μM menadione. Purified MEKK1 was treated with either 10 mM DTT (‘D’) or 10 mM ascorbate (‘A’) for 30 min, then MEKK1 activity was measured by in vitro kinase assay. Menadione-inhibited MEKK1 was not re-activated by exposure to ascorbate in vitro. (D) Wild-type catalytic domain MEKK1 was isolated from untreated cells (white bars) or cells treated with 250 μM menadione (grey bars). Purified MEKK1 was incubated with or without 20 mM dimedone for 1 h at room temperature. DTT at 10 mM was added to the indicated samples, and incubation continued for additional 30 min. The buffer containing the treatments was removed from the kinase immobilized on chitin beads, and activity was measured by using an in vitro kinase assay. A representative of three independent experiments is shown. Dimedone did not interfere with re-activation of menadione-inhibited MEKK by DTT.