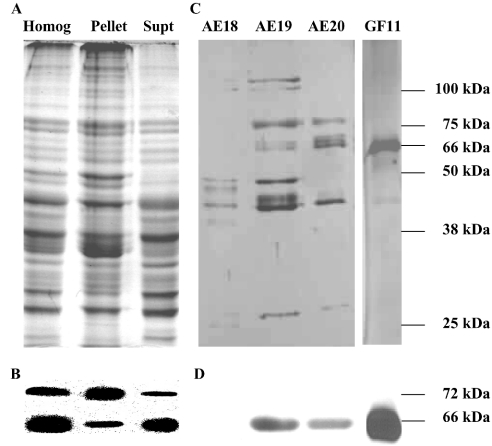
Figure 1. Lobster MO has two HMGR proteins.
(A) Samples (approx. 20 μg of protein) of a whole MO homogenate (Homog) and its 100000 g pellet (Pellet) and supernatant (Supt) were prepared from freshly dissected tissue, and analysed immediately by SDS/PAGE (Coomassie Blue staining). (B) The SDS/polyacrylamide gels in (A) were analysed with Western blots using a Psm-HMGR antiserum and showed the presence of two immunoreactive protein bands. The more abundant 66 kDa protein was found predominantly in the Supt fraction, whereas the less abundant 72 kDa protein was predominantly in the Pellet. (C) Proteins in the supernatant were purified by anion-exchange chromatography and the HMGR activity was detected in fractions 18–20 (AE18–AE20). These fractions were pooled and further purified by gel filtration and HMGR activity was highest in fraction 11 (GF11). Aliquots of fractions AE18–AE20 and GF11 were analysed by SDS/PAGE (silver staining). (D) Only the 66-kDa protein was detected by Western blotting in the fractions separated. Molecular masses of the marker proteins are shown on the right-hand side of the SDS/polyacrylamide gel and the calculated masses of the immunoreactive bands are given for the Western blots. The Figure is representative of the results obtained in approx. ten experiments.