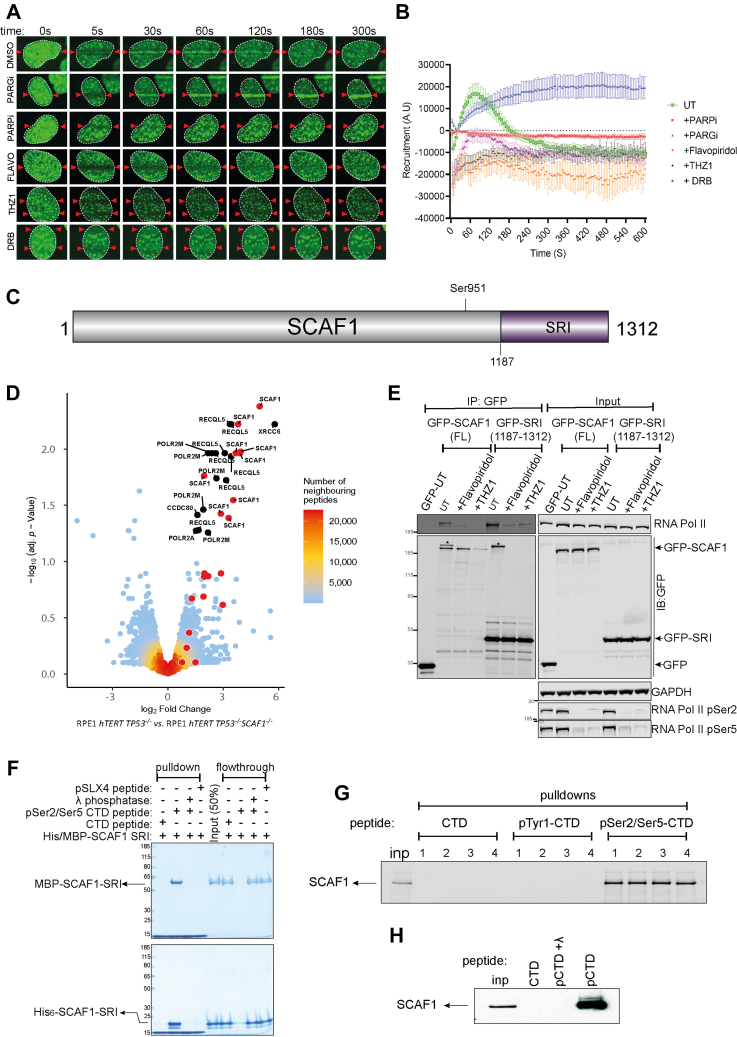
Fig. 5.
SCAF1 interacts with the Ser2/Ser5-phosphorylated CTD of RNAPII. A, BrdU-sensitized U-2 OS Flp-In T-REx cells stably expressing GFP-tagged SCAF1, pre–incubated with DMSO, olaparib (5 μM; PARPi), PDD00017273 (0.3 μM; PARGi), flavopiridol (10 μM), THZ-1 (10 μM) or DRB for 1 h were micro-irradiated with a 405 nm laser and imaged at the times indicated. B, same as (A), except that cells stably expressing GFP–SCAF1 were subjected to spot micro–irradiation (405 nm), and spot intensities were quantitated. Data represents the mean ± SEM of two independent experiments: >50 micro–irradiated cells per point. C, schematic diagram showing the domain organization of SCAF1. D, lysates of RPE1 hTERT TP53−/− cells parental cells and RPE1 hTERT TP53−/−SCAF1−/− cells were subjected to immunoprecipitation with in-house sheep anti-SCAF1 antibodies (3 biological replicates). Proteins were eluted from beads, trypsinized, and after TMT labeling, samples were pooled and injected on an UltiMate 3000 RSLCnano System coupled to an Orbitrap Fusion Lumos Tribrid Mass Spectrometer. A volcano plot representing SCAF1 interactors is shown. Dots in red (also with White border to differentiate from the peptide density) indicate SCAF1 peptides. Mass spectrometry raw data were uploaded to ProteomeXchange via jPOSTrepo with the identifier PXD041201. Data analysis scripts and annotated spectra (66) are available from Zenodo under https://doi.org/10.5281/zenodo.10581731. E, U-2 OS Flp-In T-REx cells expressing GFP-SCAF1, or GFP-SCAF1-SRI domain were subjected to immunoprecipitation with anti-GFP antibodies and precipitates were subjected to western blotting with the antibodies indicated (“IP-GFP”). Input cell extracts were analyzed in parallel. F, a biotinylated peptide containing three heptad repeats from the POL2RA CTD (CTD peptide), or the corresponding peptide that was phosphorylated at Ser2 and Ser5 in each heptad (pCTD) incubated or not with lambda phosphatase, was immobilized on streptavidin beads. A peptide corresponding to phospho-Ser1238 from SLX4 was used as a control. Beads were incubated with MBP-SCAF1-SRI or His6-tagged SRI expressed in bacteria. Beads were washed and subjected to SDS-PAGE and Coomassie staining. Both bead and supernatant “flowthrough” fractions are shown. G, the immobilized CTD and pS2/S5-CTD peptides were incubated with extracts of U-2 OS cells; after washing, precipitates were subjected to western blotting with SCAF1 antibodies. A CTD peptide where Tyr1 in each heptad was phosphorylated (pY1-CTD) was used as a control. H, same as (E), expect that the pS2/S5-CTD peptides were pre-treated or not with lambda phosphatase.