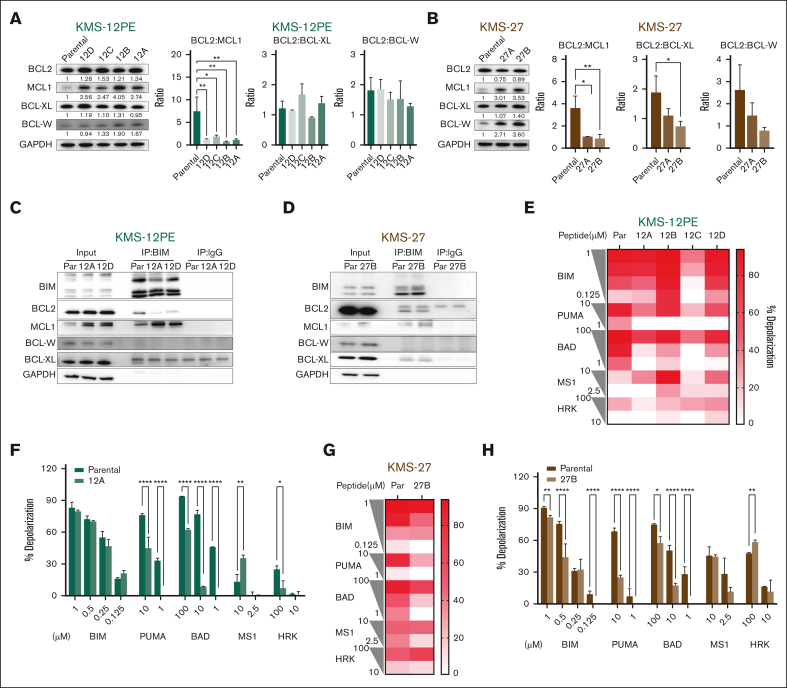
Figure 2.
Increased expression of antiapoptotic proteins characterizes the acquisition of venetoclax resistance. (A-B) Western Blot (WB) analysis of BCL-2, MCL-1, BCL-XL, and BCL-W in parental cells and clones of KMS12PE model (A) and KMS27 model (B). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Protein expression densitometry values were calculated using ImageJ. Ratios of BCL-2 vs other antiapoptotic proteins in parental and resistant clones are also illustrated. Error bars represent the standard deviation (SD) of at least duplicate results. (C) Immunoprecipitation (IP) for BIM in parental cells and clones 12A and 12D of KMS12PE model, followed by western blot for BIM, BCL2, MCL-1, BCL-W, and BCL-XL. GAPDH was used as a loading control. (D) IP for BIM in parental cells and clone 27B of KMS27 model. (E-H) BH3 profiling was performed on parental cells and resistant cells using a plate-based BH3 profiling assay and several doses of indicated peptides. Each experiment was performed in triplicate. The heat map for panels E,G represents mean of % depolarization from 1 experiment performed in triplicate in all indicated cells. The bar graph for panels F,H represents percentage of depolarization in parental and representative clones (clone 12A in panel F and clone 27B in panel H). Two-way analysis of variance (ANOVA) test was used to calculate statistical significance. ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. BAD, BCL2 and BCL-XL dependency; HRK, BCL-XL dependency; MS1, MCL1 dependency; PUMA/BIM, promiscuous peptides.