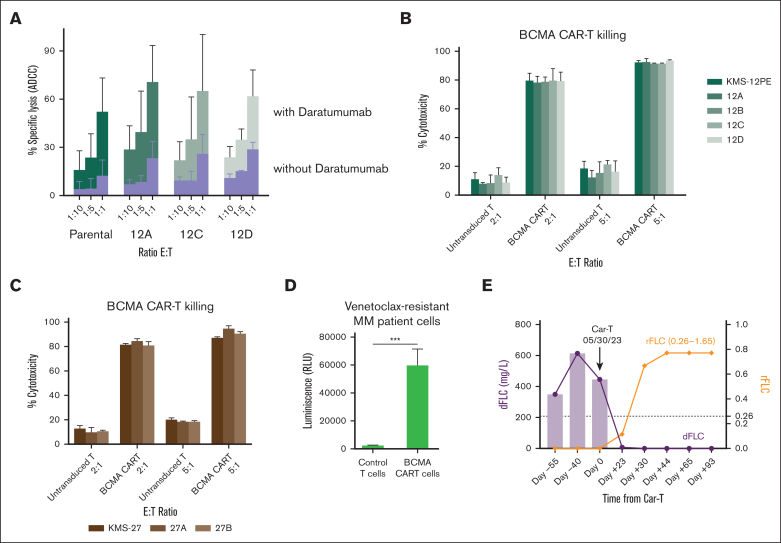
Figure 5.
Antibody-based and cellular immunotherapies are effective against venetoclax-resistant cells. (A) NK cell–mediated ADCC with or without daratumumab (1 μg/mL) in venetoclax-resistant clones compared with parental cells (KMS12PE model). (B-C) KMS12PE (B) and KMS27 (C) parental and resistant clones were incubated with either untransduced T cells or BCMA CAR-T cells for 4 hours at the effector-to-target ratio of 2:1 and 5:1. Killing was assessed by LDH release using LDH-Glo Cytotoxicity Assay kit. The percentage of target cell cytotoxicity was calculated using 10% Triton X-100 as a control. Data represent mean of 3 experiments performed in triplicates. (D) BCMA CAR-T cells were incubated with CD138+ primary cells isolated from a bone marrow aspirate of a patient with t(11;14) MM progressing on venetoclax. (E) Changes in the difference between involved and uninvolved serum-free light chains (dFLC) and κ/λ FLC ratio (rFLC) of the patient with venetoclax resistance before and after CAR-T cell therapy.