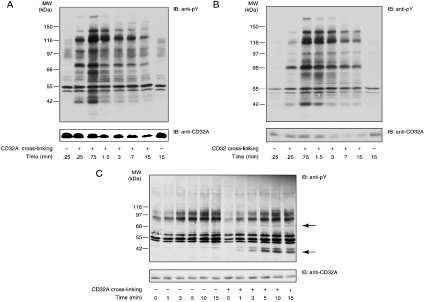
Figure 1. Tyrosine phosphorylation and receptor degradation induced after CD32A cross-linking in neutrophils, U-cytoplasts and isolated plasma membranes.
After a 10 min incubation with 1 mM iPr2P-F, CD32A was cross-linked on human neutrophils (A) or cytoplasts (B) at 20×106 cells/ml. The cells or the cytoplasts were incubated with IV.3 (2 μg/ml) for 5 min at room temperature, followed by the addition of goat F(ab′)2 anti-mouse IgG (20 μg/ml) at 37 °C for the time periods indicated. Samples were probed with an anti-phosphotyrosine (anti-pY) or anti-CD32A antibody. (C) CD32A was cross-linked on neutrophil membranes as described in the Materials and methods section for the time periods indicated. Samples were probed with an anti-pY or anti-CD32A antibody. IB, immunoblot. The two major substrates whose tyrosine phosphorylations are enhanced are indicated with arrows.