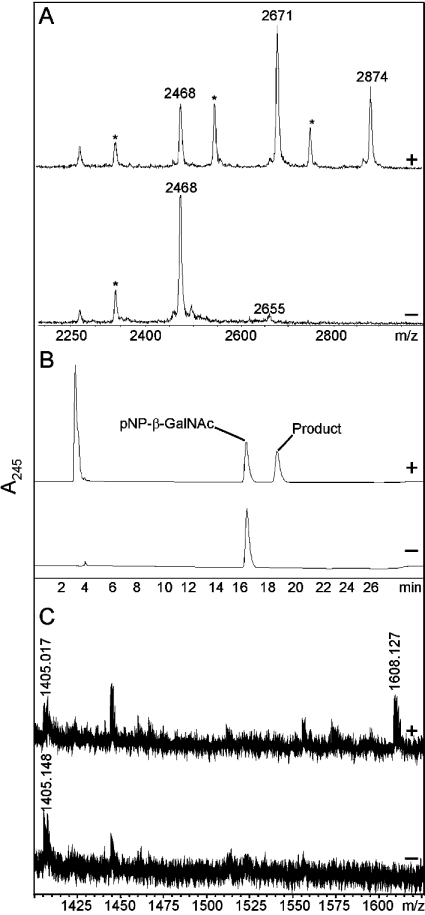
Figure 3. Characterization of the reaction products of Drosophila α4GT1.
(A) MALDI–TOF-MS of dabsyl-βGNβGN (m/z 2468) with Affi-Gel-enriched α4GT1, incubated either for 2 h in the presence of UDP-GalNAc (upper panel) or overnight in the absence of UDP-GalNAc (lower panel). Note that all dabsyl-glycopeptides were subjected to partial laser-induced removal of the distal ring giving rise to peaks (*) m/z 132 smaller than the main substrate or product peak; otherwise the detected peaks correspond to molecular ions of the form [M+H]+. (B) Reversed-phase HPLC analysis of p-nitrophenyl-β-N-acetylgalactosaminide after incubation overnight with Affi-Gel-enriched α4GT1 in the presence (upper panel) and absence (lower panel) of UDP-GalNAc. (C) MALDI–TOF-MS analysis of a core arthro-glycosphingolipid after incubation in the presence (upper panel) and absence (lower panel) of UDP-GalNAc with culture supernatant of Pichia transformed with α4GT1.