Abstract
Recurrent episodes of neurological dysfunction and white matter lesions in a young adult raise suspicion for multiple sclerosis (MS). However, occlusive retinopathy, hearing loss and absence of CSF oligoclonal bands are atypical for MS and should make the clinician consider an alternative diagnosis. We describe a man with hearing loss, visual signs and symptoms, and an accumulating burden of brain lesions, who was treated for a clinical diagnosis of MS for nearly two decades. Genetic testing revealed a unifying diagnosis.
Keywords: Multiple sclerosis, Sickle cell disease, HbSC disease, Whole exome sequencing, Hemoglobinopathy, Brain-eye-ear disease
Introduction
Recurrent episodes of neurological dysfunction and white matter lesions in a young adult raise suspicion for multiple sclerosis (MS). However, occlusive retinopathy, hearing loss and absence of CSF oligoclonal bands are atypical for MS and should make the clinician consider an alternative diagnosis. We describe a man with hearing loss, visual signs and symptoms, and an accumulating burden of brain lesions, who was treated for a clinical diagnosis of MS for nearly two decades. Genetic testing revealed a unifying diagnosis.
Case Report
A 24-year old Hispanic man with sickle cell trait, hypertension and cocaine use presented with unilateral hearing loss. Brain MRI revealed multiple lesions ‘concerning for demyelination’ (Fig. 1a). MR angiography and CSF studies were unremarkable. He was started on interferon beta-1a. Over the next 15 years, he experienced contralateral hearing loss (requiring cochlear implants), episodic dizziness, fatigue, sensory and visual symptoms. Subsequent brain MRIs showed new T2-hyperintense and persistently enhancing lesions (Fig. 1b). Ophthalmologic examination at age 36 revealed bilateral proliferative retinopathy with ‘sea fan’ neovascularization and extensive nonperfusion in the peripheral retina (Fig. 2). Bedside vestibular exam, bithermal air calorics, and rotary chair testing were significant for severe bilateral vestibular weakness. Immunologic assessment was notable for depressed CD8 T-cells (132 cells/uL) and elevated CD19 cells (758 cells/uL), IgG (1,842 mg/dL) and IgA (782 mg/dL) levels. Whole exome sequencing (WES) identified compound heterozygous pathogenic variants of the HBB gene in trans (c.20A > T and c.19G > A), diagnostic for HbSC disease.
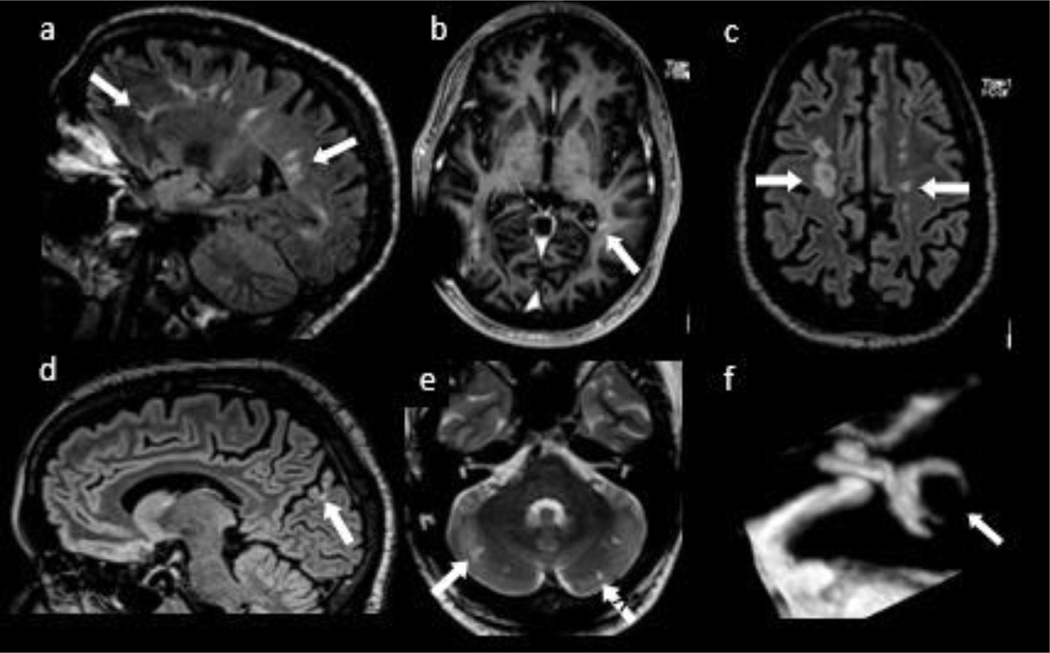
Fig. 1.
MRI sequences of the brain including sagittal FLAIR (a) shows white matter lesions in a periventricular distribution, some of which are perpendicular to the ventricular axis, but were not ‘broad based’. Volumetric T1-weighted MRI with contrast (b) demonstrates enhancement in the left peritrigonal white matter that persisted for many months. Axial FLAIR postcontrast (c) shows multiple lesions predominantly in the centrum semiovale in a linear configuration resembling a “rosary” pattern suggestive of deep border-zone infarcts. A occipital cortex lesion seen on sagittal FLAIR sequence (d) demonstrates encephalomalacia, favoring vascular injury. Multiple linear hyperintensities on T2WI (e) involving bilateral cerebellar hemispheres, were consistent with small infarcts. Constructive interference in steady state (CISS) sequence of the left labyrinth (f) shows lack of normal signal of the semicircular canals, suggestive of labyrinthitis ossificans.
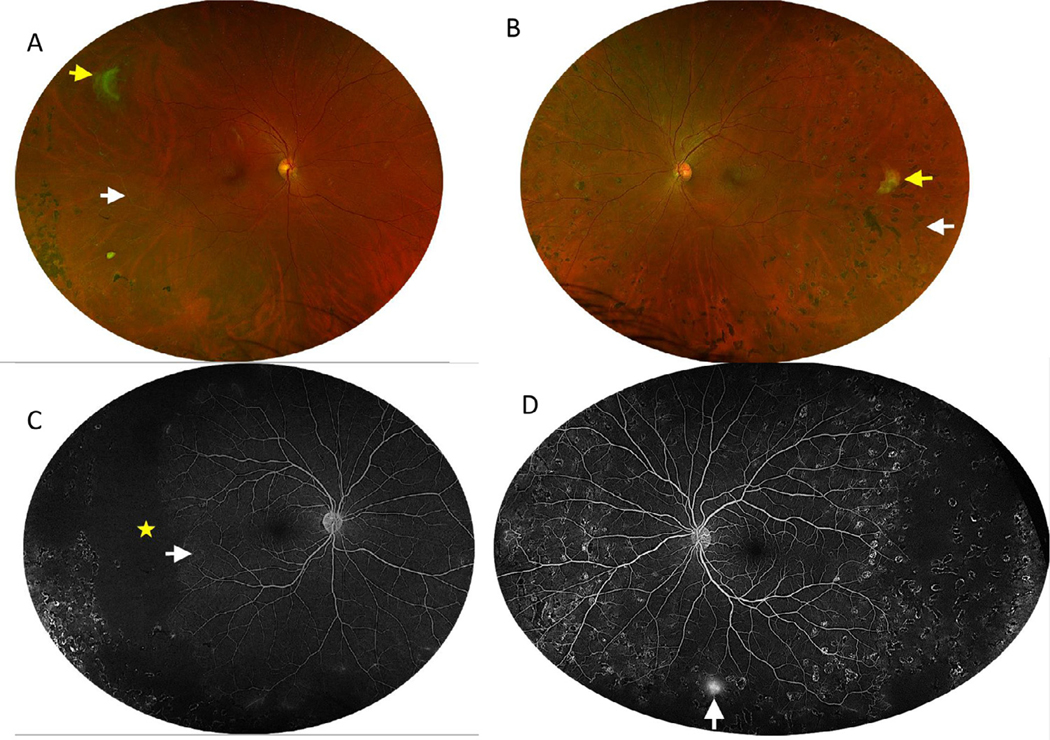
Fig. 2.
A, B: Optos Fundus photos of the right and left eye, respectively. A: The right eye demonstrates a fully regressed superotemporal sea fan neovascular complex (yellow arrow) with inferotemporal vascular sclerosis (white arrow) and far temporal laser scars. B: The left eye demonstrates a fully regressed temporal sea fan neovascular complex (yellow arrow), temporal vascular sclerosis (white arrow), and 360 degrees of laser scars. Fig. 2C, D: Optos ffuorescein angiography (FA) images of the right and left, respectively. C: Mid-phase FA images of the right eye demonstrate extensive temporal nonperfusion (yellow star), arteriovenous anastomosis and vascular remodeling in the temporal perfused retina (white arrow), and staining from prior laser scars. D: Optos FA of the left eye demonstrates staining from prior laser scars, peripheral vascular remodeling, and inferior peripheral neovascularization (white arrow).
Discussion
Brain-eye-ear (BEE) diseases1 include Susac syndrome, Vogt-Koyanagi-Harada disease, Cogan syndrome, Bechet disease, adenosine deaminase 2 deficiency, Eales disease and multiple sclerosis.1–3 Our patient’s symptoms were initially attributed to demyelination, however, in retrospect, visual impairment was due to the retinopathy, which is seen in 70% of HbSC cases,4 and hearing loss was likely due to labyrinthitis ossificans (Fig. 1f), seen in 29% of cases.4 His retinal changes were not diagnostic early in the disease course, but with time achieved a characteristic appearance for occlusive retinopathy of HbSc disease, emphasizing the importance of ophthalmologic re-evaluations over time. Radiographic infarcts (Fig 1d,e) and ‘rosary’ pattern5 of white matter injury are consistent with cerebrovascular disease, though their extent is unusual for HbSC, and likely reffect the compounding effect of extensive cocaine use.6 Periventricular lesions (‘Dawson fingers’) (Fig. 1a) are suggestive of demyelination but lack the ‘broad base’ required to satisfy Dawson’s own definition of these lesions as “wedge-shaped areas with a broad base to the ventricle”.7 Importantly, periventricular lesions perpendicular to ventricles can also be seen in vascular disease.8 To our knowledge, enhancing lesions have not been reported in HbSC and may represent subacute stroke or persistent vascular injury (Fig. 1b). Our patient’s abnormal immunologic parameters may reffect a state of chronic inffammation due to hemoglobinopathy.9,10
In summary, hemoglobinopathy should be considered on the differential of ‘BEE’ diseases, especially in the setting of microvascular occlusive retinopathy and hearing loss due to labyrinthitis ossificans. A formal ophthalmologic examination is warranted in any patient with an uncertain neurologic diagnosis in whom visual loss is a key manifestation, as many ‘MS mimickers’ have characteristic ophthalmologic findings. An unbiased WES approach may yield the diagnosis in a chronic-relapsing neurologic condition even when a genetic cause is not strongly suspected. In our case, suspicion of hemoglobinopathy would have allowed us to make the diagnosis more cost-effectively with hemoglobin electrophoresis.
Acknowledgments
Dr. Wallach has served as a consultant for Biogen and Genentech; she has received fellowship support from the National MS Society. Dr. Modi serves as a consultant for: Alimera, Allergan, Genentech, Thea, and Zeiss. Dr. Shepherd is founder and advisor to MICroStructure Imaging (MICSI), a startup for image post-processing and denoising. Dr. Scher has received financial compensation from Abbvie, BMS, Eli Lilly, Novartis, Pfizer, UCB, Janssen, and Sanofi. Dr. Kister served on advisory boards for Biogen and Genentech and received consulting fees from Roche and research support for investigator-initiated grants from Sanofi Genzyme, Biogen, EMD Serono, National MS Society, and Guthy Jackson Charitable Foundation.
Footnotes
Disclosures
The remainder of the authors, including MJB, DC, RE, CZ, AN, BS and CC report no disclosures.
Declaration of Competing Interest
None.
References
- 1.Triplett JD, Buzzard KA, Lubomski M, et al. Immune-mediated conditions affecting the brain, eye and ear (BEE syndromes). J Neurol Neurosurg Psychiatry 2019;90 (8):882–894. [DOI] [PubMed] [Google Scholar]
- 2.Tanatar A, Karadag SG, Sozeri B, et al. ADA2 deficiency: case series of five patients with varying phenotypes. J Clin Immunol 2020;40(2):253–258. [DOI] [PubMed] [Google Scholar]
- 3.Wagner W, Fehrmann A. Association of retinal vasculitis (Eales’ disease) and Meniere-like vestibulocochlear symptoms. Eur Arch Otorhinolaryngol 2006;263(2):100–104. [DOI] [PubMed] [Google Scholar]
- 4.Lionnet F, Hammoudi N, Stojanovic KS, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica 2012;97(8):1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangla R, Kolar B, Almast J, Ekholm SE. Border zone infarcts: pathophysiologic and imaging characteristics. Radiographics 2011;31(5):1201–1214. [DOI] [PubMed] [Google Scholar]
- 6.Bachi K, Mani V, Jeyachandran D, Fayad ZA, Goldstein RZ, Alia-Klein N. Vascular disease in cocaine addiction. Atherosclerosis 2017;262:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson JW. The histology of disseminated sclerosis. Edinburgh Med J 1916;17(4):229–241. [Google Scholar]
- 8.Lv A, Zhang Z, Fu Y, Yan Y, Yang L, Zhu W. Dawson’s fingers in cerebral small vessel disease. Front Neurol 2020;11:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Azevedo JTC, Malmegrim KCR. Immune mechanisms involved in sickle cell disease pathogenesis: current knowledge and perspectives. Immunol Lett 2020;224: 1–11. [DOI] [PubMed] [Google Scholar]
- 10.Cherif-Alami S, Hau I, Arnaud C, et al. Serum immunoglobulin levels in children with sickle cell disease: a large prospective study. J Clin Med 2019;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]