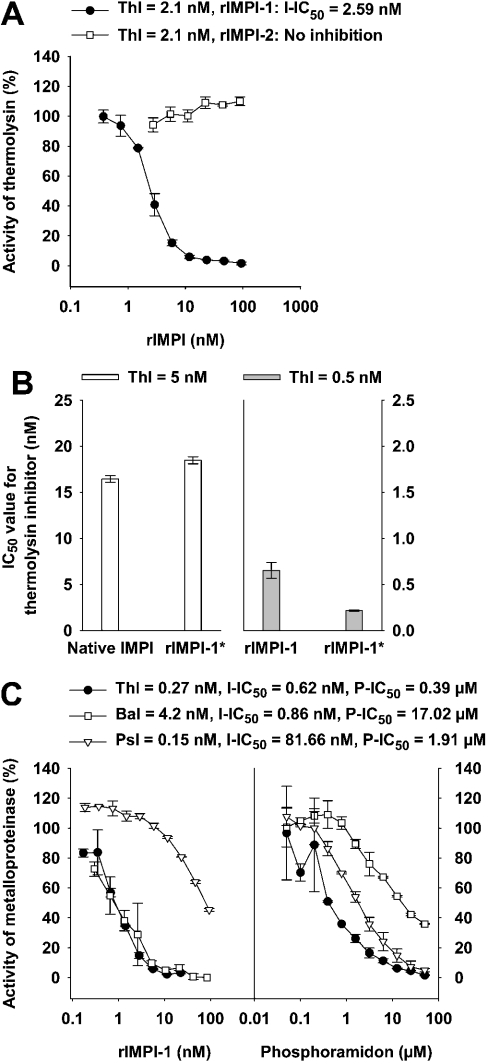
Figure 6. Inhibitory activity of native IMPI, rIMPI-1, rIMPI-1*, rIMPI-2 and phosphoramidon against bacterial metalloproteinases belonging to the gluzincin family.
For each assay, positive controls were performed and the proteinases were added at concentrations that provided similar proteolytic activity (Thl, thermolysin; Bal, bacillolysin; Psl, pseudolysin). The resulting proteinase activity was defined as 100%. The IC50 values for tested inhibitors (I-IC50 for IMPI and P-IC50 for phosphoramidon) were determined in the presence of inhibitors, which were serially diluted 3-fold and preincubated for 10 min with the proteinase before initiating the reaction by substrate addition. (A) Thermolysin activity was monitored in the presence of rIMPI-1 or rIMPI-2 using the MMP-1/MMP-9 substrate assay (assay I). (B) Comparison of the IC50 values for native IMPI and rIMPI-1* (both isolated on thermolysin–Sepharose columns), and comparison of IC50 values for rIMPI-1 (isolated on Ni2+-chelating Sepharose column) and rIMPI-1* (purified additionally on a thermolysin–Sepharose column) for thermolysin activity using the MMP-1/MMP-9 substrate assay (assay II). (C) Thermolysin, bacillolysin and pseudolysin activities were analysed in the presence of rIMPI-1 and phosphoramidon using the Azocoll assay.