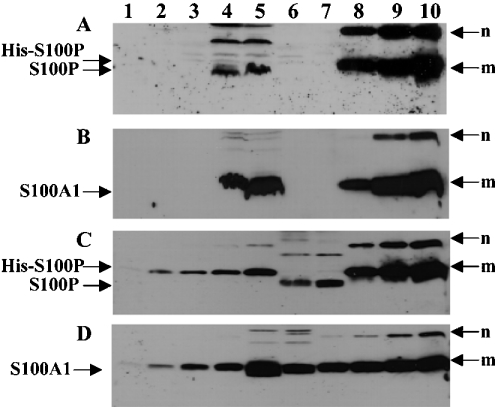
Figure 3. Western blotting for S100A1 and S100P proteins in extracts of cell lines.
(A, B) Protein (60 μg) of each cell lysate of neuroblastoma cell lines SH-EP (lane 1), SH-SY-5Y (lane 2), SK-N-AS (lane 3), breast carcinoma cell lines, MDA MB-231 (lane 4), MCF-7 (lane 5) and the human normal B lymphocyte cell line, Colo 720 L (lane 6) and recombinant proteins, S100A1 (1 μg in lane 7 of A; 0.3, 1.0 and 3 μg in lanes 8, 9 and 10 of B), His-tagged S100P (1.0 μg in lane 7 of B; 0.3, 1.0 and 3 μg in lanes 8, 9 and 10 of A) were separated by SDS/PAGE (15% gel) and transferred on to Immobilon membranes. For semi-quantification, His-tagged S100P (C), 10, 20, 40, 80, 160, 320, 640 and 1280 ng were loaded in lanes 1, 2, 3, 4, 5, 8, 9 and 10 respectively; S100A1 (D), 10, 20, 40, 80, 160, 320, 640 and 1280 ng were loaded in lanes 1, 2, 3, 4, 7, 8, 9 and 10 respectively. MCF7 cell lysate (60 μg) was loaded in lane 6 (C) and lane 5 (D). MDA MB-231 cell lysate (60 μg) was loaded in lane 7 (C) and lane 6 (D). The membranes were incubated with either mouse anti-S100P (A, C) or rabbit anti-S100A1 (B, D) and bound antibody detected as described in the Experimental section. m, monomer; n, SDS-resistant multimers of the recombinant proteins, His-S100P (A, C) and S100A1 (B, D). The higher-molecular-mass bands in lanes 4 and 5 of (A), lanes 6 and 7 of (C) are the multimers of endogenous non-His-tagged S100P.