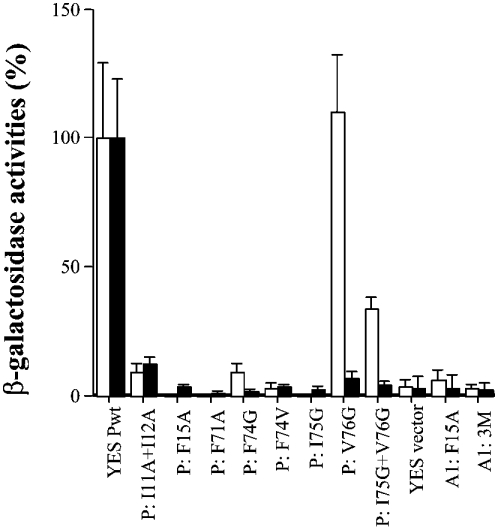
Figure 6. Effect of mutations on S100P homodimerization and heterodimerization in the yeast two-hybrid system.
The yeast strain L40 was co-transformed with the DNA constructs in pairs (bait+prey) and the transformants were grown in selected agar plates. A total of six clones were randomly chosen from each of two independent transformations of each pair of DNA constructs and their β-galactosidase activities were determined as described in the Experimental section. The relative binding affinities of bait, S100A1wt (Lex A1wt) (white) or S100Pwt (Lex Pwt) (black) to preys, S100Pwt (YES Pwt) or S100P mutants (P:=S100P) I11A+I12A, F15A, F71A, F74G, F74V, I75G, V76G, I75G+V76G or S100A1 mutants (A1:=S100A1), F15A and 3M: L11H+F15A+F71L triple mutations are expressed as the percentages of the β-galactosidase activities of Lex A1wt+YES Pwt for heterodimerization or Lex Pwt+YES Pwt for homodimerization. S100P self-association data are taken from Zhang et al. [29].