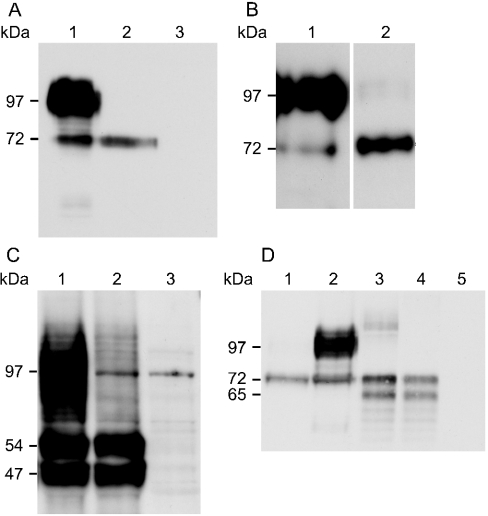
Figure 4. Western-blot analysis of Slc11a1 glycosylation patterns.
(A) Extracts (10 μg) from the stable macrophage cell lines WT3 (lane 1) and MUT12 (lane 2) expressing EGFP-tagged wild-type and mutant Slc11a1 respectively, and from untransfected RAW264.7 cells (lane 3) were separated by SDS/PAGE (10% gel). Immunoblotting was performed using an anti-GFP rabbit polyclonal antibody (1:5000) and visualized following a 30 s exposure. Positions of the 72 kDa precursor species and the 97 kDa standard that is flanked by a broad smear of fully glycosylated mature wild-type Slc11a1 are shown on the left. (B) 5 μg of WT3 (lane 1) and 15 μg of MUT12 (lane 2) extracts were immunoblotted as in (A) and visualized following a 10 min exposure to facilitate detection of low levels of mature mutant protein. No staining of untransfected RAW264.7 cells was apparent at this exposure. (C) COS7 cells were transiently transfected with 3×FLAG-tagged wild-type (lane 1) and mutant (lane 2) Slc11a1 and non-recombinant vector (lane 3). Protein was extracted and 10 μg resolved by SDS/PAGE (10% gel), 48 h post-transfection. Immunoblotting was performed using an anti-FLAG monoclonal antibody (1:400). (D) Comparison of EGFP-tagged Slc11a1 expression in RAW and COS7 cells. Extracts (10 μg) from MUT12 (lane 1), WT3 (lane 2) and COS7 cells transiently transfected for 24 h with Slc11a1wt-EGFP (lane 3), Slc11a1mut-EGFP (lane 4) and non-recombinant pEGFP-N1 (lane 5) were resolved by SDS/PAGE (10% gel). Immunoblotting was performed as in (A). Positions of the 47 and 54 kDa (C) and 65 and 72 kDa (D) precursor species and the 97 kDa standard band are shown on the left.