Abstract
Wild waterbirds sampled July 2006–September 2009 in Mongolia were tested for antibodies to avian influenza (AI) virus with the use of a commercially available blocking enzyme-linked immunosorbent assay. Antibodies were detected in 25% (572/2,282) of tested birds representing 26 species, and all antibody-positive samples were from 12 species in the orders Anseriformes and Charadriiformes. The highest antibody prevalence was in Ruddy Shelducks (Tadorna ferruginea; 61.7%; n = 261; 95% confidence interval [CI] 55.8–67.6%), Whooper Swans (Cygnus cygnus; 38.4%; n = 242; 95% CI 32.3–44.5%), Swan Geese (Anser cygnoides; 15%; n = 127; 95% CI 8.6–21.4%), Bar-headed Geese (Anser indicus; 13%; n = 738; 95% CI 10.3–15.1%), and Mongolian Gulls (Larus mongolicus; 3.9%; n = 255; 95% CI 1.3–6.5%). There was no significant temporal or spatial variation in the presence of antibodies in the sampled species. However, Bar-headed Geese and Mongolian Gulls showed spatial variation in antibody prevalence in 2007 and 2008, respectively. Our study provides insights into the hatch year waterbirds’ exposure to AI virus at their natal and molting sites in Mongolia.
Keywords: Antibodies, avian influenza virus, bELISA, Mongolia, serology, wild waterbirds
Wild waterfowl are considered the main reservoir of all subtypes of avian influenza (AI) viruses. With the use of real-time reverse transcription–polymerase chain reaction (RT-PCR) and virus isolation, nine low-pathogenic AI viruses were detected from 2,139 oropharyngeal or cloacal swabs from live and dead birds and fecal samples from single-species flocks collected at 43 locations across Mongolia from 2005 through 2008 (Spackman et al., 2009). H12N3 virus was isolated from a Bar-headed Goose (Anser indicus), a Whooper Swan (Cygnus cygnus), and two Ruddy Shelducks (Tadorna ferruginea). H13N6 and H16N3 viruses were isolated from two individual Black-headed Gulls (Chroicocephalus ridibundus). A Red-crested Pochard (Rhodonessa rufina) and two Mongolian Gulls (Larus mongolicus) were positive for H3N6 and H13N6 viruses, respectively. Likewise, highly pathogenic AI viruses of H5N1 subtype have been isolated from four Bar-headed Geese, nine Whooper Swans, three Common Goldeneyes (Bucephala clangula), and two Ruddy Shelducks, which were found as carcasses in Mongolia between May and July in 2005–2010 (Sakoda et al., 2010). Because of seasonal variation in prevalence and the transitory nature of AI virus shedding, surveillance based on virus detection assays, such as RT-PCR and virus isolation, often require large sample sizes and repeated sampling to identify populations in which AI virus is circulating. Serologic assays for detection of antibodies to AI virus potentially provide an alternative approach to identify species that are naturally infected with these viruses (Brown et al., 2009), and can provide insight into the epidemiology of AI virus in wild bird populations (Brown et al., 2010). Herein, we report the results of serological testing for AI virus antibodies in multiple waterbird species sampled in Mongolia from 2006 to 2009.
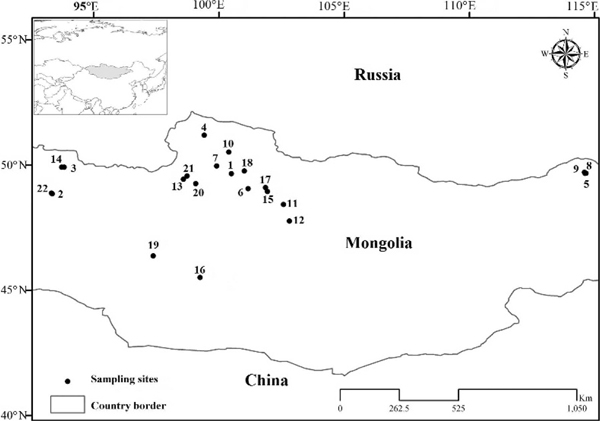
Wild birds were captured between July and September at 22 sampling sites from 2006 through 2009 (Fig. 1, Table 1). Birds were captured with the use of mist nets, herding, or spotlighting and were identified to species. Individuals were classified based on plumage characters as after-hatch-year (AHY) or hatch-year (HY) birds representing the respective year’s brood. All HY Mongolian Gulls were estimated to be >5–8 wk old, whereas HY birds of other species were estimated to be >10 wk of age. At most of the sampling sites, waterbirds were predominantly represented by nonbreeding groups; therefore, HY birds were underrepresented in these sampled species. However, exceptions include Mongolian Gulls (sampled on their natal sites), Ruddy Shelducks (sampled at molting sites), and Great Cormorants (Phalacrocorax carbo; sampled at a premigratory staging site). All captured birds were weighed and a blood sample taken from the jugular vein; ≤0.3 ml blood was collected per 100 g body weight. Birds were marked with neck collars or metal rings as appropriate to species to prevent resampling in a given year. Samples were placed in serum separator tubes, centrifuged within 6 hr of collection, and serum was aliquoted into cryotubes and frozen at −20 C for up to 1 mo, and −80 C thereafter. Serum samples were tested for antibodies directed against the nucleoprotein of type A influenza virus with the use of a commercially available blocking enzyme-linked immunosorbent assay (bELISA) kit (FlockCheck AI MultiS-Screen Antibody Test Kit, IDEXX Laboratories, Westbrook, Maine, USA) in accordance with manufacturer’s instructions.
Figure 1.
Location of serum sampling sites in Mongolia from 2006 through 2009: 11= Achmagg Nuur (49°39′N, 100°30′E); 21=Airag Nuur (48°51′N, 93°21′E); 31=Baga Nuur (49°55′N, 93°49′E); 42,3,4=Darkhad Valley (51°12′N, 99°25′E); 51=Delger Nuur (49°43′N, 114°36′E); 64=Doroo and Deed Tsagaan Nuur (49°3′N, 101°10′E); 71,2,3,4=Erhel Nuur (49°58′N, 99°54′E); 81=Khaichiin Tsagaan Nuur (49°41′N, 114°39′E); 91=Khorin Tsagaan Nuur (49°40′N, 114°37′E); 103=Khovsgol Nuur (50°31′N, 100°24′E); 113,4=Khunt Nuur (48°26′N, 102°35′N); 121,2,3,4=Ogii Nuur (47°46′N, 102°49′E); 132,3=Tsangiyn Dalai Nuur (49°16′N, 99°4′E); 141=Sharbart Nuur (49°55′N, 93°43′E); 152,3,4=Sharga Nuur (48°57′N, 101°56′E); 161=Small lake Southeast of Boontsagaan Nuur (45°31′N, 99°15′E); 172,3=Tsegeen Nuur (49°6′N, 101°52′E); 182,3,4=Tsengel Nuur (49°46′N, 101°1′E); 191=Taigam Nuur (46°22′N, 97°22′E); 201=Tunamal Nuur (49°26′N, 98°35′E); 211=Ulaan Nuur (49°34′N, 98°44′E); 221=Zost Nuur (48°53′N, 93°19′E). Sites sampled listed in bold were tested for both antibodies with a commercial blocking enzyme-linked immunosorbent assay (bELISA), and avian-influenza-virus genetic material by reverse transcription–polymerase chain reaction or viral isolation; the rest were tested only with bELISA. Superscripts 1, 2, 3, and 4 identify sites sampled in 2006, 2007, 2008, and 2009, respectively.
Table 1.
Comparison of prevalence of antibodies (Ab) to avian influenza (AI) viruses in after-hatch-year (AHY) and hatch-vear (HY) wild waterbird species from Mongolia. All birds were tested for antibodies with a commercial blocking enzyme-linked immunosorbent assay (bELISA), and tested for AI virus genetic material by reverse transcription-polymerase chain reaction (RT-PCR) or viral isolation (VI).
| Speciesa | n | Time of sampling | RT-PCR/VIb +/total (%) | bELISA Ab+/total (%) | AHYc Ab+/total (%) | HYc Ab+/total (%) |
|---|---|---|---|---|---|---|
|
| ||||||
| 2006 | ||||||
| Great Cormorant (Phalacrocorax carbo) | 5 | August and October | 0/5 (0) | 0/5 (0) | 0/1 (0) | 0/4 (0) |
| Ruddy Shelduck (Tadorna ferruginea) | 4 | July-August | 1/2 (50) | 1/4 (25) | 0/2 (0) | 1/2 (50) |
| Swan Goose (Anser cygnoides) | 28 | July | 0/22 (0) | 4/28 (14.3) | 4/17 (24) | 0/11 (0) |
| Whooper Swan (Cygnus cygnus) | 12 | August | 1/9 (11) | 2/12 (25) | 3/12 (25) | – |
| 2007 | ||||||
| Bar-headed Goose (Anser indicus) | 118 | July | 3/118 (2.5) | 12/118 (10) | 12/118 (10) | |
| Bean Goose (Anser fabalts) | 21 | July | 4/21 (19) | 8/21 (38) | 8/21 (38) | – |
| Great Cormorant (Phalacrocorax carbo) | 70 | September | 1/70 (1.4) | 0/70 (0) | 0/1 (0) | 0/69 (0) |
| Ruddy Shelduck (Tadorna ferruginea) | 4 | July | 1/4 (33) | 1/4 (33) | 1/3 (33) | 0/1 (0) |
| Ruddy Shelduck (Tadorna ferruginea) | 28 | August | 3/28 (10.7) | 10/28 (34) | 10/18 (17.2) | 0/10 (0) |
| Swan Goose (Anser cygnoides) | 17 | July | 0/17 (0) | 4/17 (23.5) | 4/17 (23.5) | – |
| Whooper Swan (Cygnus cygnus) | 120 | July-August | 7/120 (5.8) | 41/120 (34) | 41/120 (34) | – |
| 2008 | ||||||
| Bar-headed Goose (Anser indicus) | 313 | July | 8/313 (2.5) | 38/313 (12) | 38/313 (12) | – |
| Bean Goose (Anser fabalis) | 69 | July | 3/69 (4.3) | 43/69 (62) | 43/69 (62) | – |
| Great Cormorant (Phalacrocorax carbo) | 52 | September | 12/82 (14.6) | 0/82 (0) | 0/17 (0) | 0/65 (0) |
| Mongolian Gull (Larus mongolicus)d | 135 | July-August | 1/135 (0.74) | 10/135 (7.4) | 7/15 (46) | 3/120 (2.5) |
| Ruddy Shelduck (Tadorna ferruginea)e | 149 | August | 6/149 (4) | 86/149 (57.7) | 84/117 (72) | 2/32 (6.2) |
| Swan Goose (Anser cygnoides) | 101 | July | 18/101 (17.8) | 18/101 (17.8) | 18/101 (17.8) | – |
| Whooper Swan (Cygnus cygnus) | 86 | July-August | 2/86 (2.3) | 29/86 (33.7) | 29/86 (33.7) | – |
| 2009 | ||||||
| Bar-headed Coose (Anser indicus) | 309 | July | 0/309 (0) | 45/309 (15) | 45/299 (15) | 0/10 (0) |
| Grey Heron (Ardea cinerea) | 14 | July | 0/14 (0) | 0/14 (0) | – | 0/14 (0) |
| Mongolian Cull (Larus mongolicus) | 126 | July | 0/126 (0) | 2/126 (1.6) | – | 2/126 (1.6) |
| Ruddy Shelduck (Tadorna ferruginea) | 265 | August | 4/265 (1.5) | 162/265 (61) | 162/245 (66) | 0/20 (0) |
| Swan Goose (Anser cygnoides) | 126 | July | 0/126 (0) | 19/126 (15) | 19/126 (15) | – |
| Whooper Swan (Cygnus cygnus) | 54 | July | 0/54 (0) | 28/54 (52) | 25/54 (52) | – |
Other samples (no. antibody positive/no. sampled) included: 2006, Black-headed Gull (Chroicocephalus ridibundus; 1/1), Broad-billed Sandpiper (Limicola falcinellus; 0/1), Northern Lapwing (Vanellus vanellus, 0/2), Tundra Swan (Anser columbianus; 1/1); 2007, Black-throated Loon (Gavia arctica; 0/1), Great-crested Grebe (Podiceps cristatus; 0/1), Graylag Goose (Anser anser; 0/1), Mongolian Gull (Larus mongolicus; 0/3), Pacific Golden-Plover (Pluvialis fulva; 0/1), White-winged Scotor (Melanitta fusca; 1/2); 2008, Common Merganser (Mergus merganser; 1/1), Northern Shoveler (Anas clypeata, 1/1); 2009, Bean Goose (Anser fabalis, 0/1), Gadwall (Anser strepera; 0/3), Graylag Goose (Anser anser; 1/3), Northern Shoveler (Anas clypeata; 1/1), Northern Pintail (Anas acuta; 1/1), Tundra Swan (Anser columbianus; 1/1).
RT-PCR testing of cloacal swab samples collected in 2006–2007 as reported by Spackman et al. (2009). Samples collected in 2009 were only screened with viral isolation and tested for antibodies.
Dashes (−) indicate no data for species/category.
Only two samples from July sampling, negative for Ab.
Only one sample included from May sampling, negative for Ab.
We assessed temporal and spatial differences in antibody prevalence within species across same age classes and sites, with the use of chi-square tests in SPSS statistical software version 9.0 (SPSS Inc., Chicago, Illinois, USA). For an unbiased comparison of the prevalence across species or years at each site, we only tested species that were sampled at two or more sites and included ≥5 individuals per site. The adjusted Wald method was used to estimate 95% confidence intervals (CIs) for the estimates of antibody prevalence (Agresti and Coull, 1998).
In total, 2,282 wild birds belonging to six orders, eight families, and 26 species were sampled. The corresponding virus isolation and RT-PCR results from these birds have been reported (Spackman et al., 2009; Table 1). Antibodies to AI virus were detected in 572 individuals (25%, 95% CI 22.6–26.2%). Positive samples were detected in 12 species, all in the orders Anseriformes and Charadriiformes (Table 1). The prevalence of antibodies to AI virus was 16.7% (n=54, 95% CI 6.2–27.2%), 19.7% (n=390, 95% CI 15.7–23.7%), 24.5% (n=935, 95% CI 21.7–27.3%), and 29% (n=897, 95% CI 25.9–31.9%) in 2006, 2007, 2008, and 2009, respectively. Within the well-represented species, there was no temporal variation in antibody prevalence within a site. Significant spatial differences were only detected for Mongolian Gulls sampled in 2008 and Bar-headed Geese sampled in 2008 and 2009 (Table 2). Variation in the age structure of the sample cohorts for each species restricted valid comparison of antibody prevalence between HY and AHY birds. Among Anseriformes, AHY predominated, with large numbers of birds positive for antibodies to AI virus. However, sampled populations of Mongolian Gulls (Charadriiformes) and Great Cormorants (Pelecaniformes) were biased to HY birds, and were found to have low prevalence or no antibodies, respectively.
Table 2.
Avian influenza A virus antibody-positive (Ab+) numbers of birds sampled by species and location 2007–2009 in Mongolia.
| Sampled sitesa |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 |
6 |
7 |
4 |
11 |
13 |
4 |
18 |
||||||||||
| Species | Year | n | Ab+ | n | Ab+ | n | Ab+ | n | Ab+ | n | Ab+ | n | Ab+ | n | Ab+ | n | Ab+ |
|
| |||||||||||||||||
| Bar-headed Goose (Anser indicus) | 2007 | 82 | 7 | – | – | – | – | – | – | – | – | 11 | 1 | 25 | 4 | – | – |
| Ruddy Shelduck (Tadorna ferruginea) | 2007 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 18 | 10 |
| Whooper Swan (Cygnus cygnus) | 2007 | 35 | 9 | – | – | – | – | – | – | – | – | 23 | 9 | 43 | 17 | 15 | 6 |
| Bar-headed Goose (Anser indicus) | 2008 | 93 | 6 | – | – | 109 | 21 | – | – | – | – | 4 | 0 | 105 | 10 | – | – |
| Mongolian Cull (Anser indicus) | 2008 | – | – | – | – | 53 | 1 | 64 | 2 | – | – | – | – | – | – | 14 | 6 |
| Ruddy Shelduck (Tadorna ferruginea) | 2008 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 116 | 85 |
| Whooper Swan (Cygnus cygnus) | 2008 | – | – | – | – | 38 | 17 | – | – | – | – | – | – | 35 | 9 | 9 | 2 |
| Bar-headed Goose (Anser indicus) | 2009 | 76 | 10 | 9 | 5 | 17 | 3 | – | – | 43 | 8 | – | – | 145 | 17 | 19 | 2 |
| Mongolian Cull (Larus mongolicus) | 2009 | – | – | – | – | 65 | 2 | – | – | 59 | 0 | – | – | – | – | – | – |
| Ruddy Shelduck (Tadorna ferruginea) | 2009 | – | – | 82 | 50 | 157 | 100 | – | – | – | – | – | – | – | – | 22 | 11 |
| Whooper Swan (Cygnus cygnus) | 2009 | – | – | – | – | 14 | 8 | – | – | – | – | 5 | 3 | 25 | 13 | – | – |
4: Darkhad valley, 6: Doroo and Deed Tsagaan Nuur, 7: Erhel Nuur, 10: Khovsgol Nuur, 11: Khunt Nuur, 13: Tsangiyn Dalai Nuur; 16: Sharga Nuur, 18: Tsengel Nuur; Dashes indicate no birds sampled. Site numbers as given in Figure 1.
Antibody prevalence estimates and species-specific prevalence reported herein are consistent with trends from West and Central Asia and the Middle East (Fereidouni et al., 2010), with no detectable antibodies in Grey Herons (Ardea cinerea) or Great Cormorants and high prevalence in Anseriformes. The latter is consistent with patterns observed in Europe (Arenas et al., 1990; Astroga et al., 1994; De Marco et al., 2003), and North America (Brown et al., 2010) among wintering waterbirds. The prevalence of AI virus in a population is highly variable and is dependent on host species, season, geographic location and age. The absence of antibodies from particular hosts and localities could be accounted for by insufficient sampling, whereas differences in the timing of sampling could have resulted in variation in prevalence between different species.
Our study provides insights into the exposure of HY birds to AI virus at their natal as well as molting sites. Mongolia is a breeding ground for many sampled species within the orders Anseriformes and Charadriiformes (May–June). Many waterbird species are long-distance migrants, breed in (mixed species) colonies, and congregate in large numbers in staging areas outside the breeding season (July–September; Braunlich et al., 2002) except for Mongolian Gulls, which breed in July(Nyambayar et al., 2007; M. Gilbert, pers. obs.). In mixed-species colonies, viruses may readily be transmitted between individuals from the same or different species. Extensive surveillance studies of wild ducks in the northern hemisphere have revealed high prevalence of influenza virus primarily in HY birds, with a peak in virus recovery in early fall prior to southbound migration (Krauss etal., 2004; Fouchier et al., 2007; Wallensten et al., 2007). Similar patterns have been observed in northern Europe and Siberiaprior to winter migration (Okazaki et al., 2000). Given this timing of AI transmission, our sampling period (July–September) should have coincided with the increased seasonal transmission of AI virus; however, HY Great Cormorants, Ruddy Shelducks, and Mongolian Gulls showed either no or low prevalence of antibodies to AI.
Within the antibody-positive HY birds, there are two explanations: first, presence of maternally derived antibodies and second, antibody response to AI virus exposure. For example, presence of antibody positives among HY Mongolian Gulls could either represent exposure to AI virus at the natal site, or presence of maternally derived antibodies in HY birds. However, as maternally derived antibodies to AI virus decrease to zero in 3–4 wk posthatching (Maas et al., 2011), and the age of sampled Mongolian Gulls was greater than 5 wk old, then the antibodies detected in this age class most likely represent exposure to virus at the natal site. Transfer of AI virus specific maternal antibodies is known to catabolize within 2 wk posthatching in Kittiwakes (Rissa tridactyla; Staszewski et al., 2007); thus, chicks should be susceptible to infection thereafter.
Second, based on the corresponding viral isolation/RT-PCR data, the results show consistent patterns of seroconversion at natal site (Velarde et al. 2010). First, fledgling Ruddy Shelducks may have acquired antibodies during exposure at staging areas, which is supported by negative RT-PCR in these individuals in 2008 (see Table 1). These results indicate that our sampling was either conducted before the peak transmission period or that the spread of AI virus is very slow in sampled Ruddy Shelducks. Alternatively, these individuals had been recently infected but had not yet seroconverted at the time of testing. In Great Cormorants, virus was detected with the use of RT-PCR in individuals without detectable antibodies, suggesting that 1) either Great Cormorants had been recently infected and had not yet seroconverted, 2) Great Cormorants do not produce detectable levels of antibodies, 3) the RT-PCR results were false positives, 4) the bELISA was unable to detect AI virus antibodies produced by the cormorants. Most serological tests are developed for use in domestic poultry and vary in their ability to detect antibodies in nongallinaceous wild birds accurately. It is possible that serologic tests are ineffective in Great Cormorants, which also might have a reduced antibody response compared to other avian species.
With the use of antibody and RT-PCR prevalence data, our study highlights that 1) HY birds are being exposed to AI virus at natal and staging areas in Mongolia, albeit at low levels, and 2) the importance of age-specific data in serologic studies for understanding of the underlying epidemiologic dynamics of AI infections in the population.
We thank the Mongolian Ministry of Nature Environment and Tourism, and the Veterinary and Animal Breeding Agency for support. Thanks to Losolmaa Jambal and Enkhtuvshin Shiilegdamba for technical assistance. Funding was provided by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Department of Health and Human Services, under contract HHSN2662007 00007C. The opinions expressed herein are those of the authors and do not necessarily reflect the views of any of the funding agencies. D. Joly was supported by a gift from a private donor to the Wildlife Conservation Society.
LITERATURE CITED
- AGRESTI A, AND COULL B. 1998. Approximate is better than ‘‘exact’’ for interval estimation of binomial proportions. American Statistician 52: 119–126. [Google Scholar]
- ARENAS A, CARRANZA J, PEREA A, MIRANDA A, MALDONADO A, AND HERMOSO M. 1990. Type A influenza viruses in birds in southern Spain: Serological survey by enzyme-linked immunosorbent assay and haemagglutination inhibition tests. Avian Pathology 19: 539–546. [DOI] [PubMed] [Google Scholar]
- BRAUNLICH A. 2002. Birding in Central Asia: An introduction to Mongolia. Bulletin Oriental Bird Club 35: 62–70. [Google Scholar]
- BROWN JD, STALLKNECHT DE, BERGHAUS RD, LUTTRELL MP, VELEK K, KISTLER W, COSTA T, YABSLEY MJ, AND SWAYNE DE. 2009. Evaluation of a commercial blocking enzyme-linked immunosorbent assay to detect avian influenza virus antibodies in multiple experimentally infected avian species. Clinical and Vaccine Immunology 16: 824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN JD, LUTTRELL MP, UHART MM, FERREYRA H, ROMANO MM, RAGO MV, AND STALLKNECHT DE. 2010. Antibodies to Type A influenza virus in wild waterbirds from Argentina. Journal of Wildlife Diseases 46: 1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MARCO MA, FONI GE, CAMPITELLI L, RAFFINI E, DI TRANI L, DELOGU M, GUBERTI V, BARIGAZZI G, AND DONATELLI I. 2003. Circulation of influenza viruses in wild waterfowl wintering in Italy during the 1993–1999 period: Evidence of virus shedding and seroconversion in wild ducks. Avian Diseases 47: 861–866. [DOI] [PubMed] [Google Scholar]
- FEREIDOUNI SR, WERNER O, STARICK E, BEER M, HARDER TC, AGHAKHAN M, MODIRROUSTA H, AMINI H, MOGHADDAM MK, BOZORGH-MEHRIFARD MH, AKHAVIZADEGAN MA, GAIDET N, NEWMAN SH, HAMMOUMI S, CATTOLI G, GLOBIG A, HOFFMANN B, SEHATI ME, MASOODI S, Dodman T, Hagemeijer W, Mousakhani S , and METTENLEITER TC. 2010. Avian influenza virus monitoring in wintering waterbirds in Iran, 2003–2007. Virology Journal 7: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOUCHIER RAM, MUNSTER VJ, KEAWCHAROEN J, OSTERHAUS ADME, AND KUIKEN T. 2007. Virology of avian influenza in relation to wild birds. Journal of Wildlife Diseases 43: S7–S14. [Google Scholar]
- KRAUSS S, WALKER D, PRYOR SP, NILES L, CHENGHONG L, HINSHAW VS, AND WEBSTER RG. 2004. Influenza A viruses of migrating wild aquatic birds in North America. Vector-Borne and Zoonotic Diseases 4: 177–189. [DOI] [PubMed] [Google Scholar]
- MAAS R, ROSEMA S, VAN ZOELEN D, AND VENEMA S. 2011. Maternal immunity against avian influenza H5N1 in chickens: limited protection and interference with vaccine efficacy. Avian Pathology 40: 87–92. [DOI] [PubMed] [Google Scholar]
- NYAMBAYAR B, BRÄUNLICH A, TSEVEENMYADAG N, SHAR S, AND GANTOGS S. 2007. Conservation of the critically endangered east Asian population of Dalmatian Pelican Pelecanus crispus in western Mongolia. Birding Asia 7: 68–74. [Google Scholar]
- OKAZAKI K, TAKADA A, ITO T, IMAI M, TAKAKUWA H, HATTA M, OZAKI H, TANIZAKI T, NAGANO T, NINOMIYA A, DEMENEV VA, TYAPTIRGANOV MM, KARATAYEVA TD, YAMNIKOVA SS, LVOV DK, AND KIDA H. 2000. Precursor genes of future pandemic influenza viruses are perpetuated in ducks nesting in Siberia. Archives of Virology 145: 885–893. [DOI] [PubMed] [Google Scholar]
- SAKODA Y, SUGAR S, BATCHLUUN D, ERDENE-OCHIR T, OKAMATSU M, ISODA N, SODA K, TAKAKUWA H, TSUDA Y, YAMAMOTO N, KISHIDA N, MATSUNO K, NAKAYAMA E, KAJIHARA M, YOKOYAMA A, TAKADA A, SODNOMDARJAA R, AND KIDA H. 2010. Characterization of H5N1 highly pathogenic avian influenza virus strains isolated from migratory waterfowl in Mongolia on the way back from the southern Asia to their northern territory. Virology 406: 88–94. [DOI] [PubMed] [Google Scholar]
- SPACKMAN E, SWAYNE DE, GILBERT M, JOLY DO, KARESH WB, SUAREZ DL, SODNOMDARJAA R, DULAM P, AND CARDONA C. 2009. Characterization of low pathogenicity avian influenza viruses isolated from wild birds in Mongolia 2005 through 2007. Virology Journal 6: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STASZEWSKI V, GASPARINI J, MCCOY KD, TVERAA T, AND BOULINIER T. 2007. Evidence of an interannual effect of maternal immunization on the immune response of juveniles in a long-lived colonial bird. Journal of Animal Ecology 76: 1215–1223. [DOI] [PubMed] [Google Scholar]
- VELARDE R, CALVIN SE, OJKIC D, BARKER IK, AND NAGY E. 2010. Avian influenza virus H13 circulating in ring-billed gulls (Larus delawarensis) in southern Ontario, Canada. Avian Diseases 54(s1): 411–419. [DOI] [PubMed] [Google Scholar]
- WALLENSTEN A, MUNSTER VJ, LATORRE-MARALEF N, BRYTTING M, ELMBERG J, FOUCHIER RAM, FRANSSON T, HAEMING PD, KARLSSON M, LUNDKVIST A, OSTERHAUS ADME, Stervander M, Waldenstrom J , and Olsen B. 2007. Surveillance of influenza A virus in migratory waterfowl in northern Europe. Emerging Infectious Diseases 13: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]