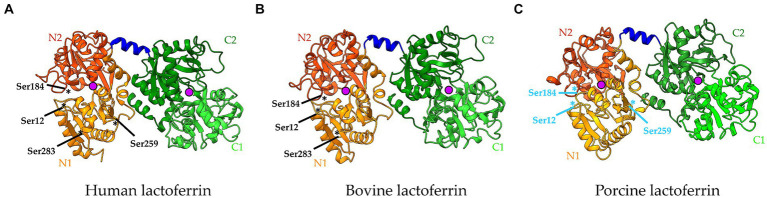
Figure 1.
Cartoon representation of the 3D structure of (A) human lactoferrin (PDB code = 1B0L), (B) bovine lactoferrin (PDB code = 1BLF), and (C) porcine lactoferrin (Uniprot ID = P14632). The lactoferrin molecule consists of two lobes, the N-lobe (highlighted in orange) and the C-lobe (highlighted in green), connected by an α-helix structure (highlighted in dark blue). Each lobe can be further subdivided into two domains: N1, N2, C1, C2. Each lobe contains an iron (Fe3+)-binding site (purple spheres). Serine residues that were previously identified as possible candidates responsible for the protease activity of lactoferrin are indicated in black, while serine residues that are putatively involved are indicated in light blue. The location of these residues is indicated with an asterisk. Figures were created using UCSF Chimera X v. 1.6.1 software.