Abstract
Background
Neuroprognostication for disorders of consciousness (DoC) after severe acute brain injury is a major challenge, and the conventional clinical approach struggles to keep pace with a rapidly evolving literature. Lacking specialization, and fragmented between providers, conventional neuroprognostication is variable, frequently incongruent with guidelines, and prone to error, contributing to avoidable mortality and morbidity.
Recent Findings
We review the limitations of the conventional approach to neuroprognostication and DoC care, and propose a paradigm entitled the Recovery of Consciousness Via Evidence-Based Medicine and Research (RECOVER) program to address them. The aim of the RECOVER program is to provide specialized, comprehensive, and longitudinal care that synthesizes interdisciplinary perspectives, provides continuity to patients and families, and improves the future of DoC care through research and education.
Implications for Practice
This model, if broadly adopted, may help establish neuroprognostication as a new subspecialty that improves the care of this vulnerable patient population.
Introduction
Disorders of consciousness (DoC) after severe acute brain injury (such as anoxia, trauma, intracranial hemorrhage, and ischemic stroke) present major clinical challenges. Neuroprognostication for DoC—which entails the evaluation of a patient's current level of consciousness and chances for a meaningful neurologic recovery—often dictates whether life-sustaining treatment (LST) is continued or withdrawn.1 Error in neuroprognostication may thus lead to avoidable mortality, morbidity, and health care costs.2 A rapidly evolving literature has explored ways of improving neuroprognostication, through scrutiny of conventional approaches,2,3 development of new technologies,4,5 and introduction of models and aids for decision making.1,6 The Neurocritical Care Society's Curing Coma Campaign has highlighted and promoted progress in this field. However, these advances have not effectively translated into clinical practice. Clinical DoC guidelines7 are inconsistently followed,8 and emerging techniques are rarely implemented, resulting in variable, outdated, and suboptimal DoC management that is prone to error.2,9 In this article, we describe the development and implementation of an innovative paradigm—entitled the Recovery of Consciousness Via Evidence-Based Medicine and Research (RECOVER) program (Figure 1)—which provides specialized, comprehensive, and longitudinal care to ensure that clinical DoC care keeps pace with developing guidelines and discoveries while improving the future of DoC care through research and education. We aim to outline a framework that, if adopted broadly, may advance the practice of neuroprognostication.
Figure 1. The Conventional Neuroprognostication Model and the RECOVER Program Model.
The conventional model of neuroprognostication care is characterized by multiple dimensions of fragmentation between providers, between disciplines, between clinical care and research, and across time. Neurologists frequently use variable approaches to neuroprognostication; specialists who interpret prognostic studies frequently do not communicate directly with those prognosticating; research (represented in green) is typically not coordinated with clinical care (represented in blue) and advanced techniques developed by research are inefficiently translated into clinical practice; and clinicians responsible for prognostication often do not provide longitudinal support beyond hospital discharge. By contrast, the RECOVER program model provides a dedicated specialized consultation service, which collects prognostic biomarkers in a systematic and evidence-based fashion, which are discussed during interdisciplinary conferences. Patients who survive beyond hospital discharge are supported longitudinally by the same team of providers. Apart from ensuring consistent evidence-based clinical care, this clinical infrastructure is leveraged to produce high-quality research data and to facilitate the translation of research discoveries into clinical practice, hence integrating clinical care (blue) and research (green) throughout. Although not represented in this figure, education is another central component of the RECOVER program, with neurology residents and trainees incorporated throughout inpatient and outpatient settings.
The Conventional Neuroprognostication Model and Its Limitations
The conventional approach to neuroprognostication typically operates as follows (Figure 1): If a patient suffers a DoC resulting from severe acute brain injury, neurologists may be consulted for neuroprognostication. Neurology consultants, often general neurologists, will typically collect a history and perform a neurologic examination. When needed, electroencephalography (EEG) and neuroimaging data are typically interpreted by neurophysiologists/epileptologists and neuroradiologists, respectively. The neurology consult team synthesizes these data and delivers their impressions to the clinical teams and patient surrogates. Patients who survive beyond discharge may not be followed by an outpatient neurologist familiar with them or may not be followed by an outpatient neurologist at all. This conventional system is similar for patients in neurocritical care units, although impressions may be formulated by neurointensivists instead of general neurologists. Although there are undoubtedly neurologists and neurointensivists who have accrued experience and expertise in prognostication through this conventional model, the model itself remains inherently limited in at least 3 domains: clinical care, education, and research.
Clinical Limitations
One challenge of the conventional model is that there exists no formalized subspecialization in neuroprognostication within neurology. Those tasked with these evaluations are typically either general neurologist consultants or neurointensivists, who are simultaneously responsible for numerous other (often more urgent) aspects of neurologic care. Limited bandwidth for neuroprognostication may contribute to the slow clinical translation of innovations produced by a rapidly evolving literature.7,10 For example, the conventional neurologic examination has been found to be insensitive for detecting consciousness (associated with a 40% misdiagnosis rate3), prompting guidelines to endorse more detailed behavioral assessments such as the Coma Recovery Scale–Revised (CRS-R).11 Neuroprognostication has been found to be prone to error immediately after brain injury, prompting guidelines to recommend deferring definitive prognostication for at least 3 days, but as many as 28 days, after brain injury.7,12 Advanced technologies such as functional magnetic resonance imaging (fMRI) may provide additional information about a patient's current level of consciousness and capacity for future neurologic recovery,4,5 prompting guidelines to endorse the clinical collection of these data.7,10 However, given competing responsibilities, some neurologists may be unfamiliar with the evolving literature and/or may not have the requisite experience or resources to implement recommended techniques, which may contribute to inconsistent guideline adherence.2,8 Moreover, limited bandwidth may preclude the serial examinations often necessary to detect consciousness,13 or the serial meetings often necessary to support and guide surrogates and families.14 Given the increasing complexity and demands of neuroprognostication, “expert consultation” has been recommended for these evaluations,15 suggesting a role for subspecialization in this field.
There are several other limitations of the conventional model that stem from its fragmentation across time, between providers, between disciplines, and between clinical care and research. The fragmentation across time—that is, discontinuity of care and inconsistent communication between the neurologists performing acute neuroprognostication and the providers caring for survivors after hospital discharge (e.g., physiatrists)—means that many neurologists may not witness the recoveries they try to predict and thus may have limited exposure to the range of possible outcomes, may not receive feedback on the accuracy of their predictions, and may not be able to counsel families based on firsthand experience with recovery. Apart from the detriments to prognostic accuracy, this lack of continuity also means that surviving patients and their families may not receive longitudinal support. Patients with robust neurologic recoveries after DoC still suffer high rates of emotional and cognitive sequelae but frequently cannot access necessary services.12 For patients who do not recover to acceptable levels of function, frequently there are no designated providers to readdress goals of care and considerations of hospice. Although different institutions, to different extents, may provide longitudinal support or interdisciplinary collaboration, such care is not widely standardized or formalized through a structured program. There is a growing demand to better integrate critical care into the broader continuum of care, and yet few models for doing so.
The conventional model is fragmented between prognosticating providers (e.g., neurologists), in that prognostic assessments of a given patient often vary widely.2,9 The cause of such variability is likely multifactorial but may reflect a lack of familiarity with guidelines, scant and variable prognostic testing, cognitive bias, and the inconsistent exposure to long-term outcomes described above.2,9,16 Such variability not only increases the odds of error but may also lead to disparities in care17 and distress patient surrogates and clinical teams who receive inconsistent messages and recommendations.
The conventional model is fragmented between disciplines. The neurology consult team often relies on other subspecialists (e.g., neurophysiologists/epileptologists and neuroradiologists) to acquire and interpret data. However, the nuance and complexity of their prognostic implications may be inconsistently communicated. There are also other disciplines critical to anticipating recovery and the psychosocial implications of LST decisions, such as physiatry, social work, physical therapy, occupational therapy, ethics, and palliative care. However, communication between the consulting neurologist and these disciplines is often limited, resulting in less comprehensive guidance and inconsistent messaging to surrogates.
Educational Limitations
Like the neurology consultants, neurology trainees may not witness the recoveries they try to predict, may inconsistently observe evidence-based and guideline-based practices, may not learn about emerging prognostic techniques, may not have the opportunity to learn from the various disciplines critical to wholistic neuroprognostication, and may not receive formalized training in discussing prognosis and LST decisions with surrogates. Trainees, therefore, may continue to adopt variable neuroprognostication practices and perpetuate the conventional model.
Research Limitations
The conventional model impedes the research necessary to advance neuroprognostication. First, if overwhelmed surrogates are approached by researchers in a manner that is insensitive or uncoordinated with clinical care, they may decline enrollment. Second, variable and suboptimal neuroprognostication practices result in heterogeneous and ungeneralizable cohorts.2 Third, several patients lack a formalized follow-up plan, and those who do follow-up may find returning for multiple clinical and research visits burdensome, impeding study retention. Fourth, the conventional clinical infrastructure is not conducive to rigorous therapeutic trials.18 Finally, when research does produce new prognostic techniques, such as advanced neuroimaging,4,5 clinical translation is often slow, as discussed above.
Overall, the conventional approach to neuroprognostication lacks the specialization necessary to keep pace with a rapidly evolving literature and is characterized by multiple dimensions of fragmentation that not only hamper care but also impede the education and research necessary for improving future care. There is a critical need for a novel approach to neuroprognostication that synthesizes and implements the multidisciplinary advances of this evolving field.
The RECOVER Program
The objective of the RECOVER program is to provide specialized, comprehensive, and longitudinal care for patients with DoC resulting from acute brain injury, using up-to-date techniques to detect, predict, and promote the recovery of consciousness while improving the future of care through education and research. To accomplish these goals, the RECOVER program seeks to provide integration across the historically fragmented dimensions of DoC care (Figure 1).
Such integration has previously been attempted in various forms. In one case, a nursing-led team provided detailed neurologic assessments, systematic prognostic biomarker collection, conferences to discuss neuroprognostication, and follow-up care.19 In response to prognostic uncertainty about DoC associated with coronavirus disease 2019 (COVID-19), some developed “coma boards,” or interdisciplinary conferences comprising neurologists, radiologists, and physiatrists, to provide input on prognosis.20 Among neurointensivists, there is a growing interest in outpatient clinics to provide longitudinal follow-up care for patients with severe brain injury.21 The RECOVER program builds on and integrates these efforts to further advance neuroprognostication and DoC care.
In this article, we outline how this paradigm has been developed and implemented at the Hospital of the University of Pennsylvania, an academic tertiary care hospital that cares for a range of acute brain injuries. We intend to provide a framework that may be used to overcome the limitations of conventional care.
Inpatient Care
Hospitalized patients are evaluated by the RECOVER program if and when primary clinical teams request a neuroprognostication consultation for DoC resulting from acute brain injury. (Clinical teams were notified of the program through departmental communications and request consultation either by contacting the service directly, or by contacting the general neurology consultants, who then redirect the request to the RECOVER service.) RECOVER consultations are typically requested after brain injuries have stabilized so that prognosis for an established injury burden can be evaluated; however, there are no timing restrictions, and requests can occur as early or as late as primary teams deem appropriate.
The RECOVER consult service is operated by dedicated neurology residents, who participate as part of a mandatory clinical rotation. Residents are supervised by a RECOVER neurologist (e.g., neurointensivist) with interest and expertise in neuroprognostication, who is committed to providing longitudinal care across inpatient and outpatient settings for patients with DoC from acute brain injury. The RECOVER consult service evaluates the patient, recommends prognostic data collection based on systematic, evidence-based, and guideline-based protocols, and helps identify and mitigate factors that impair the patient's mental status and recovery. The RECOVER consult service's focus on these patients permits the serial assessments necessary for rigorous consciousness evaluation.13 The RECOVER consultation further triggers involvement of a physiatrist and palliative care specialist who provide parallel evaluation, physical and occupational therapists who acquire serial CRS-R assessments, and a social worker who provides additional guidance and support to patients and families (Figure 2). An early priority of the consultation is to engage with each patient's surrogate and/or family, to describe the purpose of the consultation, to establish general expectations for the process and timeline, to begin assessing the patient's values and goals, and to begin developing a trusting relationship.
Figure 2. Interdisciplinary Inpatient Consultation.

The inpatient RECOVER consultation requires coordination between disciplines. A neuroprognostication consult not only involves a neurologist but also triggers the parallel involvement of physiatry, palliative care, physical and occupational therapy (PT/OT), and social work. Depending on the consensus reached during interdisciplinary conferences about a patient's prognosis and discussion with the patient's surrogates, life-sustaining treatment (LST) is either continued for the purpose of pursuing restorative goals or withdrawn for the purpose of pursuing palliative goals, which prompts the involvement of different team members. Discharge planning for patients who survive beyond hospital discharge is facilitated by a social worker familiar with facilities and resources available to patients with brain injury.
When appropriate, the RECOVER program will recommend and facilitate the acquisition of advanced diagnostic and prognostic techniques. Although techniques such as fMRI are endorsed by clinical guidelines,7,10 implementing these technologies in the acute setting is often challenging.4 The RECOVER consult service helps determine when such techniques are justified and coordinates them with clinical care. Emerging prognostic techniques may include technologies such as resting state, stimulus-based, and task-based fMRI and EEG.4,5
At regular intervals, the RECOVER program holds interdisciplinary conferences to discuss patients evaluated by the consult service, with representation from several relevant disciplines (Figure 2). At our institution, these conferences are held weekly, but frequency elsewhere may vary based on clinical volume. During the conference, for each patient evaluated, each representative discusses their perspective. The consulting neurologist presents the history, examination, and other relevant clinical data. A neuroradiologist interprets neuroimaging data, evaluating the extent of diffuse injuries such as anoxia (trialing quantitative techniques when available),15 identifying injury to arousal-related structures,22 and providing clarity about injury etiology. If advanced techniques such as fMRI have been acquired, the neuroradiologist comments on the presence of cognitive-motor dissociation23 (the willful modulation of brain activity in response to commands) or other prognostically relevant findings. Neurophysiologists/epileptologists interpret electrophysiologic data and provide systematic, evidence-based evaluation of their prognostic implications; in anoxic brain injury, for example, a consensus-based framework is used for categorizing EEG features as “reassuring” (associated with favorable outcomes), “indeterminate” (not reliably associated with outcomes), “concerning” (predictive of unfavorable outcomes in some studies), or “indicative of a poor outcome” (consistently predictive of unfavorable outcomes across studies). Physical and occupational therapists provide the results of their CRS-R evaluations, observations of recovery trends, and strategies for increasing patient arousal. A physiatrist provides a perspective informed by experience with long-term recovery and recommends interventions to optimize recovery. A social worker comments on the resources and facilities available to the patient and their family, outlines the psychosocial and socioeconomic implications of treatment paths, and provides strategies for supporting patients and families. Palliative care providers offer input on end-of-life decision making and care. An ethicist helps navigate complex dynamics with surrogates and ethical and legal challenges as they arise.
These interdisciplinary conferences serve several purposes. First, the group aims to formulate a wholistic, consensus-based prognosis, drawing on prognostic biomarkers and contextual factors to predict neurologic function, quality of life, and socioeconomic ramifications for patients and families. These discussions allow the nuance and complexities of these considerations to be communicated and permit the reconciliation of opposing data or perspectives, as interdisciplinary discourse may produce more accurate prognostic assessments.24 Such discussions also ensure that providers across disciplines offer consistent messaging to surrogates and clinical teams, avoiding the miscommunications and contradictions that may otherwise disrupt care. Second, the group aims to formulate a treatment plan that may include therapeutic interventions, strategies for optimizing neurologic recovery, and methods for supporting surrogates. Finally, leveraging the longitudinal follow-up described hereafter, conferences offer an opportunity to communicate the outcomes of previously discussed patients, so that the group can collectively learn and incorporate feedback. Even as clinicians rotate through the RECOVER consult service, these interdisciplinary conferences, along with prognostication protocols, mitigate variability across providers.
Based on the conference's conclusions, the RECOVER consult service assists clinical teams in discussions with surrogates and families, communicating prognosis and treatment options using a systematic framework.1 The way in which data are synthesized to formulate a prognosis and guide decisions is generally informed by several fundamental principles (expounded elsewhere1): Whenever feasible, multimodal prognostic biomarkers are collected to account for the imperfect predictive value of any single test; when prognostic biomarkers are discrepant, biomarkers are weighted by the quality of the data and the literature or guidelines that support them; when appropriate, time-limited trials are offered to gauge recovery trajectories (uniquely enabled by this program's infrastructure)25; and most importantly, guidance is tailored to each patient's values and wishes.1
When the outcome of these discussions favors ongoing LST and recovery optimization, the RECOVER neurologist collaborates with physiatry, physical therapy, and occupational therapy to develop treatment strategies, establish mobility goals, and provide rehabilitative interventions (Figure 2). In such cases, the RECOVER clinicians also assure patients and families that they will maintain a longitudinal relationship to support the patients' recovery. When the outcome favors the withdrawal of LST, the RECOVER neurologist collaborates with palliative care to ensure appropriate end-of-life support (Figure 2). For patients who survive to hospital discharge, a social worker familiar with facilities and resources available to patients with brain injury assists with discharge planning (Figure 2).
Postacute Care
Patients for whom LST is continued, and who survive to hospital discharge, are typically transferred to a postacute facility, such as a long-term acute care hospital (LTACH), inpatient rehabilitation facilitation (IRF), or skilled nursing facility (SNF). Neurologically, this is a critical period for 3 reasons. First, the neurologic prognosis, if still uncertain, often becomes clearer during this time—it is often while in a postacute facility that patients reach the 28 days after injury that some guidelines advise waiting to evaluate a recovery trajectory.7 Second, consciousness recovery during this time can be subtle, with some patients regaining only minute or inconsistent purposeful movements that can be easily overlooked.3 And third, neurologic complications, such as seizures and myoclonus, may arise or persist during this period. However, many postacute facilities do not have the necessary neurology resources to detect and optimize DoC recovery.
By partnering with a local LTACH and IRF, the RECOVER program provides continuity of care during this critical postacute period through a ‘RECOVER transition program’ (Figure 1). Patients evaluated by the RECOVER service are regularly reviewed for LTACH or IRF transfer eligibility by the RECOVER social worker. When appropriate, transfer eligibility is also discussed during interdisciplinary conferences. For patients transferred to the RECOVER transition program at a partnered LTACH or IRF, the RECOVER neurologist and physiatrist, who are credentialed across inpatient and postacute facilities, continue to collaborate with therapists to monitor recovery with serial CRS-R assessments and guide treatments. Leveraging continuity from the acute hospitalization, RECOVER clinicians provide more informed management of neurologic complications and maintain their relationships with patients and their families, which helps ensure that care is consistent with the patient's physiology and goals and engenders trust.
At the conclusion of the postacute facility stays, RECOVER clinicians evaluate the neurologic trajectories to revisit prognostic discussions with patients and surrogates. As needed, cases are rediscussed during interdisciplinary conferences. RECOVER clinicians help ensure that the discharge destinations are consistent with each patient's goals and prognosis, advocating for more intensive rehabilitation or hospice care as appropriate.
Outpatient Care
Patients who survive beyond the postacute phase are followed longitudinally in an outpatient RECOVER clinic operated by the same neurology residents and supervised by the same RECOVER neurologist who provided inpatient consultation, to ensure continuity (Figure 1). Appointments are preferentially held in-person but can also be attended virtually for patients for whom transport is logistically, medically, or financially prohibitive.
RECOVER clinics serve 2 primary purposes. First, RECOVER clinics address the chronic neurologic sequelae of acute brain injury. For patients with chronic DoC, RECOVER clinicians, often in collaboration with physiatry, manage symptoms such as spasticity and myoclonus and may trial stimulants as appropriate. For patients who recover more robustly, RECOVER clinicians screen for and manage the emotional and cognitive sequelae that often persist.12 Second, for patients who do not recover to acceptable levels of function, RECOVER clinics offer an opportunity to revisit discussions of prognosis and goals of care. When appropriate, RECOVER clinicians, in collaboration with palliative care, refer such patients to hospice. The interdisciplinary conferences remain a useful resource for discussing outpatients with ongoing treatment and prognostication needs and offer a venue for sharing the long-term outcomes of previously discussed patients.
Education and Research
The RECOVER program is intended not only to improve neuroprognostication care in the present but also to ensure that it continues to improve through education and research. The RECOVER program promotes education by incorporating trainees such as neurology residents, neurocritical care fellows, epilepsy fellows, neuroradiology fellows, physiatry residents, and brain injury rehabilitation fellows. Neurology residents on the consult service receive formalized education about current neuroprognostication literature and guidelines, the implementation and interpretation of emerging prognostic techniques, interdisciplinary perspectives through conference participation, and how to lead discussions of prognosis and decisions about LST with surrogates and families. Because the program helps to ensure that neurology residents maintain continuity with surviving patients in the outpatient clinic, trainees observe the continuum of recovery, manage common sequelae of DoC, and learn how to revisit discussions of prognosis and LST in the outpatient setting. By promoting education, the RECOVER program helps trainees to provide sound neuroprognostication and perhaps establish similar paradigms, wherever they ultimately practice.
The RECOVER program also offers a unique opportunity to advance and translate research. The RECOVER program helps identify inpatients with DoC and an uncertain prognosis through consultation requests; patient data are compiled in a registry, which further facilitates study screening. The RECOVER program helps ensure that research consent and data collection are appropriately coordinated with clinical care. The consistent and evidence-based care delivered by the RECOVER program helps mitigate the variability of previous studies. The RECOVER program's structured follow-up facilitates study retention, and clinic visits are coordinated with data collection on patient outcomes. And finally, the close integration of clinical care and research helps facilitate and accelerate the translation of discoveries into clinical practice. The RECOVER program is not bound to any particular prognostic techniques; as prognostic techniques continue to evolve, the specialized and interdisciplinary members of the RECOVER program will help ensure that practice evolves along with them while providing the ethics support necessary to ensure responsible implementation.
Feasibility and Implementation
We launched the RECOVER program at the Hospital of the University of Pennsylvania on August 15, 2022, and in this article, we report implementation data collected over the following 15 months, until November 15, 2023 (Figure 3). We evaluated 129 patients (mean age 56, SD = 16.5) with DoC caused by acute brain injury (averaging 9 per month): 98 (76%) with anoxic injury after cardiac arrest; 12 (9%) with ischemic stroke; 9 (7%) with intracranial hemorrhage; and 10 (8%) with other etiologies such as traumatic brain injury, meningoencephalitis, and carbon monoxide poisoning. Patients were evaluated at a median of 4.15 days (interquartile range 1.49—9.80) from hospital admission.
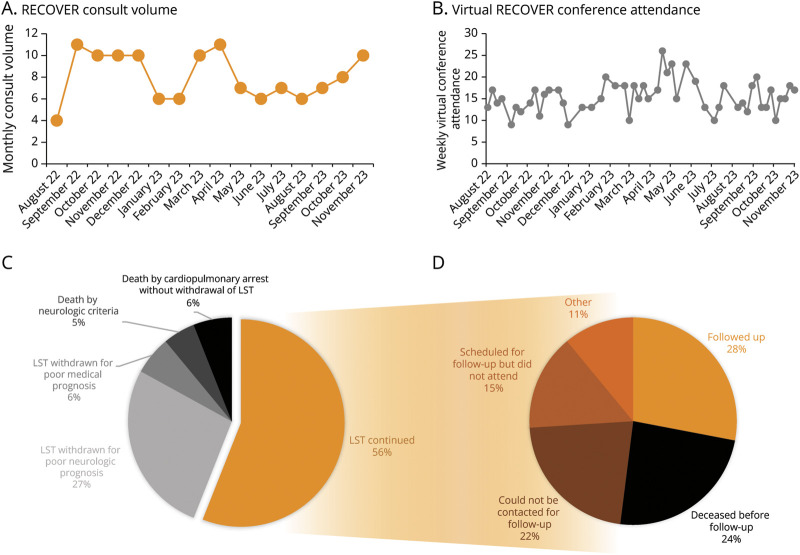
Figure 3. Feasibility and Implementation.
Data collected during the first 15 months of the RECOVER program are presented. Consult volume averaged 9 patients per month (A), and virtual interdisciplinary conference attendance averaged 16 per week in addition to in-person attendance (B), with both remaining stable over time. Outcomes from the inpatient evaluation, regarding the continuation or withdrawal of life-sustaining treatment (LST) and mortality, are presented (C). Of the patients who had LST continued and who were evaluated at least 3 months before the conclusion of the observation period, long-term outcomes regarding clinic retention are presented (D).
Advanced neuroimaging data were collected in patients with DoC who had no contraindications to an MRI scan, were sufficiently medically stable to tolerate transport and the scan, had sedatives minimized, and had surrogates who consented to the scan.4 Advanced neuroimaging (e.g., fMRI) data were collected on a preliminary basis over the first 7 months in a total of 5 patients, and more routinely over the following 8 months in an additional 9 patients. Results were interpreted and discussed during interdisciplinary conferences and relayed to clinical teams and surrogates.
A total of 32 neurology residents rotated through the consult service, in addition to other interested trainees including non-neurology residents and fellows from neurocritical care, stroke, and brain injury rehabilitation.
Weekly interdisciplinary conferences were held on 50 occasions and cancelled on 16 occasions when there were no patients to discuss. In total, 102 patients (79%) were discussed during at least one conference; of patients who were not discussed, 13 (10%) died before conference, 7 (5%) rapidly recovered consciousness before conference and did not have ongoing recovery needs, and the remaining 7 (5%) were not discussed because of logistics (e.g., time constraints). Conferences were held in a hybrid format with in-person and virtual attendance options; in-person attendance was not systematically recorded but typically ranged between 2 and 10 attendees, and virtual attendance averaged 16 attendees per conference (SD = 4). Conferences routinely included input from neurocritical care, neuroradiology, epilepsy, physiatry, palliative care, ethics, physical/occupational therapy, and social work and was further attended by other physicians and trainees depending on availability and interest.
Regarding patient outcomes, by the conclusion of the inpatient RECOVER consultation, 72 patients (56%) had LST continued, 35 patients (27%) had LST withdrawn primarily because of a poor neurologic prognosis, 8 patients (6%) had LST withdrawn primarily because of a poor medical prognosis, 6 patients (5%) died by neurologic criteria, and 8 patients (6%) died by cardiopulmonary arrest (without withdrawal of LST). Of the patients discharged to an IRF or LTACH, 50% (10 of 20) and 63% (5 of 8), respectively, were transferred to a partnered facility, where RECOVER clinicians offered continuity.
Outpatient clinics were held weekly (either virtually or in-person) and scheduled for surviving patients typically at 3 months after discharge. Of the 54 patients who completed their inpatient evaluation more than 3 months before November 15, 2023, and had LST continued, 15 (28%) followed up in clinics, where they were seen by neurology residents and the RECOVER neurologist. Of the remaining patients, 13 (24%) died before follow-up, 12 (22%) could not be contacted for follow-up, 8 (15%) were scheduled but did not attend, and the remaining 6 (11%) either remained admitted to the hospital, moved away, or did not desire follow-up.
Conclusion and Future Directions
Neurology's historical approach to neuroprognostication has struggled to keep pace with a rapidly evolving literature and is characterized by fragmentation that hampers clinical care, trainee education, and research. The RECOVER program seeks to provide integration across time, providers, and disciplines to improve neuroprognostication and DoC care more generally. In addition, the RECOVER program offers a platform for enhancing trainee education and research, which are necessary to improve the future of neuroprognostication.
There remain opportunities for optimizing clinical care within this program. For example, clinic retention may be improved by more regular contact with patients or families after discharge. Moreover, further research is necessary to establish the RECOVER program's value. Implementation science evaluating how the RECOVER program influences factors such as consistency and guideline adherence of neuroprognostication, withdrawal of LST, trainee education, length of stay, readmission rates, clinic retention, clinical translation of novel technologies, patient outcomes, and patient and family satisfaction is critical and underway. Regarding cost effectiveness, many institutions already have the personnel necessary to implement such a program, there are efforts to secure insurance coverage for advanced prognostic assessments (e.g., fMRI), and the RECOVER program may generate additional revenue (e.g., more frequent inpatient consults, postacute consults, and outpatient clinics). Nevertheless, a formal cost-effectiveness analysis of designating a specialized prognostication team, providing the requisite training, and procuring equipment for advanced techniques is an important next step.
This paradigm is intended to serve as a framework that can be adopted and adapted across institutions. For institutions capable of implementing similar paradigms, we hope to collaborate in advancing neuroprognostication and DoC care. Patients, resources, and personnel vary across institutions, and thus, the infrastructure will need to be adjusted accordingly. For institutions unable to adopt a RECOVER program (e.g., in neurologically underserved locations that may not have local expertise or prognostic tools), we hope to develop a virtual RECOVER service to provide remote evaluations and to establish a network of institutions with RECOVER programs that could serve as referral centers, which may help address neurologist shortages. Harmonization across centers not only would advance care by promoting quality, consistency, and accessibility but also could serve as a platform for multicenter trials aiming to develop prognostic biomarkers and therapeutic strategies.
Historically, the profound impact of neuroprognostication has not been matched by a proportional degree of rigor. Correcting this imbalance requires fundamentally restructuring our approach to neuroprognostication, and the RECOVER program aims to do so by providing specialization, interdisciplinary collaboration, longitudinal support, and translational research. We note that expertise in neuroprognostication does not arise naturally from existing training schemes within neurology—general neurologists typically lack training in severe brain injury while neurointensivists typically lack outpatient exposure. Given that neuroprognostication increasingly demands a unique skillset, knowledge of a rapidly growing literature, interdisciplinary collaboration, specialized techniques, and involvement across both acute and chronic settings, we suggest that the RECOVER program may eventually form the basis of a new subspecialty of Consciousness Prognostication and Recovery.
The impact of the RECOVER program on neuroprognostication is currently uncertain. It is possible that, with further validation and adoption, the program may advance the future of neuroprognostication. It is probable that this program will coordinate existing resources to optimize the current practice of neuroprognostication. However, even if these efforts fail, it is at least certain that the RECOVER program will help ensure that patients and their families are not left navigating the uncertainty of brain injury alone.
TAKE-HOME POINTS
→ Neuroprognostication for disorders of consciousness (DoC) after acute brain injury—which entails predicting recovery of consciousness and function—often dictates whether life-sustaining treatment is continued or withdrawn but remains challenging.
→ Limitations of the conventional clinical model of neuroprognostication likely contribute to inaccurate neuroprognostication and suboptimal DoC care while impeding the education and research necessary to advance the field.
→ The Recovery of Consciousness via Evidence-Based Medicine and Research (RECOVER) program is an innovative model that aims to provide specialized, comprehensive, and longitudinal care to patients with DoC resulting from acute brain injury.
→ The RECOVER program, which was launched in August 2022 and has evaluated 129 patients over the following 15 months, aims to optimize current clinical care by coordinating multidisciplinary consultation, facilitating clinical translation of emerging techniques, and providing continuity across the acute, postacute, and chronic settings while advancing the future of clinical care by promoting neuroprognostication education and research.
→ If adopted broadly, the RECOVER program model may help establish Consciousness Prognostication and Recovery as a new subspecialty within neurology to help address the unique training, expertise, and infrastructure required for neuroprognostication.
Appendix. Authors
| Name | Location | Contribution |
| David Fischer, MD | Division of Neurocritical Care, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Benjamin S. Abella, MD, MPhil | Department of Emergency Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Geoffrey D. Bass, MD, MBA | Division of Pulmonary, Allergy and Critical Care, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Jeremy Charles, MD | Department of Physical Medicine and Rehabilitation, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Stephen Hampton, MD | Department of Physical Medicine and Rehabilitation, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Catherine V. Kulick-Soper, MD | Division of Epilepsy, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Matthew T. Mendlik, MD, PhD | Department of Palliative Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Oscar J. Mitchell, MD | Division of Pulmonary, Allergy and Critical Care, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Aliza M. Narva, JD, RN | Ethics, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| William Pino, PT, DPT | Physical Therapy, Good Shepherd Penn Partners at the Hospital of the University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Morgan L. Sikandar, LCSW | Clinical Resource Management and Social Work, Hospital of the University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Saurabh R. Sinha, MD, PhD | Division of Epilepsy, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Genna J. Waldman, MD | Division of Epilepsy, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Jeffrey B. Ware, MD | Division of Neuroradiology, Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Joshua M. Levine, MD | Division of Neurocritical Care, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
Study Funding
This work was supported by grants from the National Institutes of Health and National Institute of Neurological Disorders and Stroke (R25NS06574309), the Pennsylvania Medical Society Innovation Grant, the Neurocritical Care Society Research Training Fellowship, the University of Pennsylvania University Research Foundation Grant, and the University of Pennsylvania Center for Clinical Epidemiology and Biostatistics Research Program Award.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Fischer D, Edlow BL, Giacino JT, Greer DM. Neuroprognostication: a conceptual framework. Nat Rev Neurol. 2022;18(7):419-427. doi: 10.1038/s41582-022-00644-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127-135. doi: 10.1016/j.resuscitation.2016.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;9:35. doi: 10.1186/1471-2377-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer D, Newcombe V, Fernandez-Espejo D, Snider SB. Applications of advanced MRI to disorders of consciousness. Semin Neurol. 2022;42(3):325-334. doi: 10.1055/a-1892-1894 [DOI] [PubMed] [Google Scholar]
- 5.Fischer D, Edlow BL. Coma prognostication after acute brain injury: a review. JAMA Neurol. 2024;81(4):405-411. doi: 10.1001/jamaneurol.2023.5634 [DOI] [PubMed] [Google Scholar]
- 6.Muehlschlegel S, Goostrey K, Flahive J, Zhang Q, Pach JJ, Hwang DY. Pilot randomized clinical trial of a goals-of-care decision aid for surrogates of patients with severe acute brain injury. Neurology. 2022;99(14):e1446-e1455. doi: 10.1212/WNL.0000000000200937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch Phys Med Rehabil. 2018;99(9):1699-1709. doi: 10.1016/j.apmr.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 8.Elmer J, Steinberg A, Callaway CW. Paucity of neuroprognostic testing after cardiac arrest in the United States. Resuscitation. 2023;188:109762. doi: 10.1016/j.resuscitation.2023.109762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maciel CB, Barden MM, Youn TS, Dhakar MB, Greer DM. Neuroprognostication practices in postcardiac arrest patients: an international survey of critical care providers. Crit Care Med. 2020;48(2):E107-E114. doi: 10.1097/CCM.0000000000004107 [DOI] [PubMed] [Google Scholar]
- 10.Kondziella D, Bender A, Diserens K, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27(5):741-756. doi: 10.1111/ene.14151 [DOI] [PubMed] [Google Scholar]
- 11.Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85(12):2020-2029. doi: 10.1016/j.apmr.2004.02.033 [DOI] [PubMed] [Google Scholar]
- 12.Nolan JP, Sandroni C, Böttiger BW, et al. European Resuscitation Council and European Society of intensive care medicine guidelines 2021: post-resuscitation care. Resuscitation. 2021;161:220-269. doi: 10.1016/j.resuscitation.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 13.Wannez S, Heine L, Thonnard M, Gosseries O, Laureys S. Coma Science Group collaborators. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann Neurol. 2017;81(6):883-889. doi: 10.1002/ana.24962 [DOI] [PubMed] [Google Scholar]
- 14.Weber U, Zhang Q, Ou D, et al. Predictors of family dissatisfaction with support during neurocritical care shared decision-making. Neurocrit Care. 2021;35(3):714-722. doi: 10.1007/s12028-021-01211-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajajee V, Muehlschlegel S, Wartenberg KE, et al. Guidelines for neuroprognostication in comatose adult survivors of cardiac arrest. Neurocrit Care. 2023;38(3):533-563. doi: 10.1007/s12028-023-01688-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg A, Fischhoff B. Cognitive biases and shared decision making in acute brain injury. Semin Neurol. 2023;43(5):735-743. doi: 10.1055/s-0043-1775596 [DOI] [PubMed] [Google Scholar]
- 17.Mody P, Pandey A, Slutsky AS, et al. Gender-based differences in outcomes among resuscitated patients with out-of-hospital cardiac arrest. Circulation. 2021;143(7):641-649. doi: 10.1161/CIRCULATIONAHA.120.050427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marino MH, Whyte J. Treatment trials in disorders of consciousness: challenges and future directions. Brain Sci. 2022;12(5):569-615. doi: 10.3390/brainsci12050569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson R, Davies J, Keeble TR, Perilla D, Damian M. A new model for nurse lead neuro-prognostication services in out of hospital cardiac arrest in a tertiary cardiac centre. Resuscitation. 2022;170:209-210. doi: 10.1016/j.resuscitation.2021.12.004 [DOI] [PubMed] [Google Scholar]
- 20.Waldman GJ, Thakur KT, Der Nigoghossian C, et al. Multidisciplinary guidance to manage comatose patients with severe COVID-19. Ann Neurol. 2020;88(4):653-655. doi: 10.1002/ana.25830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffa MN, Podell JE, Motta M. A change of course: the case for a neurorecovery clinic. Neurocrit Care. 2020;33(2):610-612. doi: 10.1007/s12028-020-00976-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer D, Boes A, Demertzi A, et al. A human brain network derived from coma-causing brainstem lesions. Neurology. 2016;87(23):2427-2434. doi: 10.1212/WNL.0000000000003404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondziella D, Friberg CK, Frokjaer VG, Fabricius M, Møller K. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87(5):485-492. doi: 10.1136/jnnp-2015-310958 [DOI] [PubMed] [Google Scholar]
- 24.Sloane KL, Miller JJ, Piquet A, Edlow BL, Rosenthal ES, Singhal AB. Prognostication in acute neurological emergencies. J Stroke Cerebrovasc Dis. 2022;31(3):106277. doi: 10.1016/j.jstrokecerebrovasdis.2021.106277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miranda SP, Morris RS, Rabas M, Creutzfeldt CJ, Cooper Z. Early shared decision-making for older adults with traumatic brain injury: using time-limited trials and understanding their limitations. Neurocrit Care. 2023;39(2):284-293. doi: 10.1007/s12028-023-01764-8 [DOI] [PubMed] [Google Scholar]