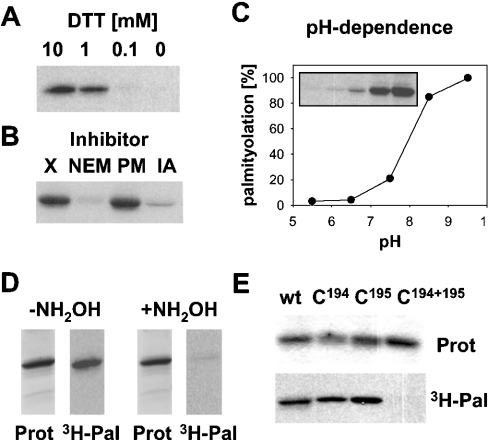
Figure 2. Palmitoylation of hYkt6 occurs on Cys194 and Cys195.
(A) DTT at the indicated concentration was added to the palmitoylation reaction. (B) N-Ethylmaleimide (NEM, 5 mM), PMSF (PM, 1 mM) and iodoacetamide (IA, 1 mM) were added to the palmitoylation reaction. X, no additions. (C) Palmitoylation reactions took place in 20 mM Pipes/120 mM KCl, pH 5.5 or 6.5, or in 20 mM Tris/120 mM KCl at pH 7.5, 8.5 or 9.5. The resulting fluorogram (inset) was quantified. Relative palmitoylation was plotted against the pH of the reaction mixture. (D) Two identical palmitoylation reactions were subjected to SDS/PAGE and Coomassie Blue staining (Prot). One part of the gel was treated with 1 M hydroxylamine (+NH2OH), the other part with 1 M Tris (−NH2OH). Gels were then processed for fluorography (3H-Pal). (E) Wild-type Ykt6 and the cysteine→serine mutants (C194, C195 and C194+195) were incubated with [3H]Pal-CoA. Samples were then subjected to SDS/PAGE, Coomassie Blue staining (Prot) and fluorography (3H-Pal). Fluorograms from three experiments were quantified to determine acylation. The Cys194→Ser mutant showed 55%, and the Cys195→Ser mutant 87%, palmitoylation relative to the wild-type (wt) protein.