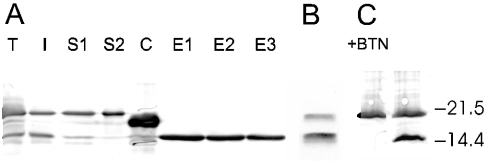
Figure 2. Expression and purification of bacterial avidin.
(A) Immunoblot showing the expression of recombinant OS-avidin within the periplasmic space of E. coli and the purification of the produced protein. Lane T shows a sample of the total E. coli lysate and lane S1 shows the soluble fraction after cell lysis. The upper band represents a non-processed OS-avidin form that still contains a signal peptide, whereas the lower band corresponds to a mature peptide without the signal. Lane I corresponds to the insoluble fraction from cell lysis and lane S2 the flow-through of the soluble fraction after 2-iminobiotin–agarose purification. In lane C, a commercial avidin standard (Belovo) is shown and, in lanes E1–E3, the OS-avidin elution fractions released at pH 4 are shown. As the Figure shows, the processed OS-avidin represents the active form since it is the only form that is not present in the flow-through fraction, and becomes eluted. (B) Eluted fraction from the purification of NS-avidin. The unprocessed and processed forms of avidin are seen. (C) Effect of biotin treatment on the total cell sample heated at 80 °C. Biotin-treated (+BTN) and -untreated samples are shown. Monomeric forms of the processed avidin are not present on the blot in the presence of biotin, whereas the non-processed form stays monomeric with and without biotin. The biotin-stabilized avidin tetramers of the processed form are not visible. Locations of molecular-mass standard bands at 21.5 and 14.4 kDa is shown (Bio-Rad Laboratories).