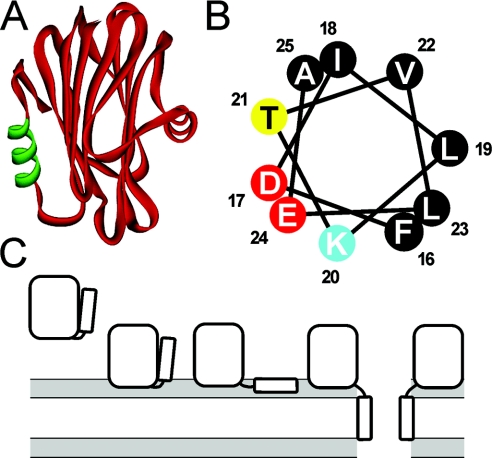
Figure 1. The three-dimensional model of Eqt-II.
(A) The three-dimensional model of Eqt-II. The N-terminal amphipathic helix comprising residues 16–25 is shown in green. (B) The helical wheel analysis of the 16–25 region. The residues are coloured according to physical properties (black, hydrophobic; yellow, polar; red, negatively charged; blue, positively charged). (C) A scheme of Eqt-II pore-formation. The pore formation involves at least four different conformational states of the toxin: a soluble form, a membrane-bound form attached to the membrane with the aromatic cluster and phosphorylcholine site, a membrane-bound non-lytic form with the N-terminal helix lying parallel to the plane of the membrane, and an oligomeric lytic form with the N-terminal helix inserted in a perpendicular orientation to the plane of the membrane as a part of the conductive pathway. The lipid–water interface is shaded grey. Reproduced from [14] with permission. © (2002) The American Society for Biochemistry and Molecular Biology.