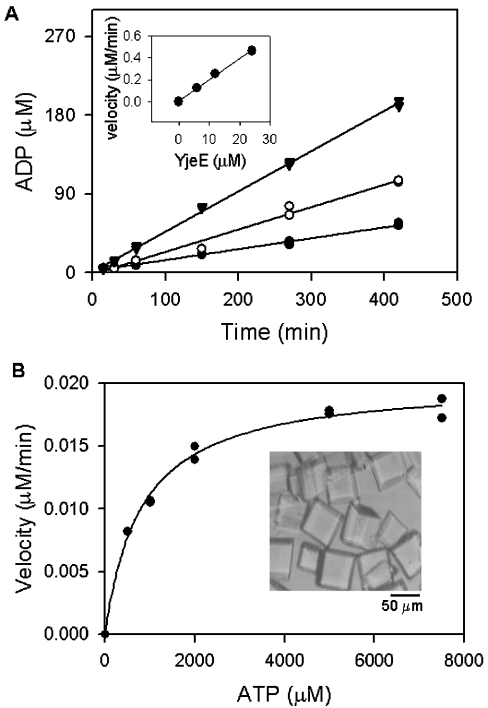
Figure 3. ATPase activity of crystalline pure H. influenzae YjeE.
Crystals of YjeE (B, inset) were prepared and dissolved as described (in the Experimental section) and ATPase activity was determined. (A) YjeE at concentrations of 6 μM (●), 12 μM (○) and 24 μM (▼) was incubated in the presence of 6 mM ATP and 10 mM MgCl2 (reaction volume, 100 μl) and 10 μl fractions were sampled at the times indicated and quenched by the addition of urea to 6 M. The amount of ADP produced was determined by HPLC as described in the Experimental section. The inset shows the initial velocity of the reaction plotted as a function of enzyme concentration; the slope represents the turnover of the enzyme under these conditions (1.2 h−1). (B) Dependence of the reaction velocity on ATP concentration. Reactions (20 μl) containing ATP (0.2–7.5 mM), YjeE (12 μM) and 10 mM MgCl2 were quenched after 7 h and analysed by HPLC to determine the rates. The data were fitted to the Michaelis–Menten equation v=kcat[E][S]/(Km+[S]) using Sigma Plot 2000 (SPSS Science, Chicago, IL, U.S.A.) and Km and kcat values were calculated to be 800 μM and 1.2 h−1 respectively.