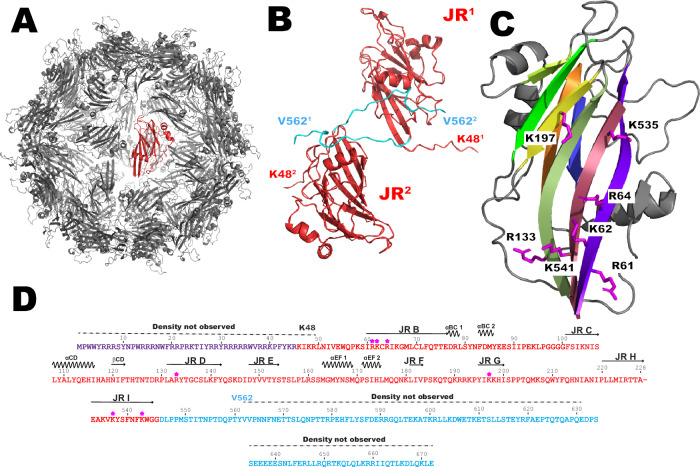
Fig. 4. Sixty LY1 jelly roll domains form the core of anellovirus particles.
A The LY1 core structure comprised of 60 jelly roll domains pack in icosahedral symmetry with one domain uniquely colored in red. B Two jelly roll domains are shown in red with the observed C-terminal domain backbone colored in cyan. The jelly roll domains are arbitrarily labeled JR1 and JR2 with the first (K48) and last (V562) observed residues for each protomer labeled with the corresponding number for clarity. C A single jelly roll domain is oriented to show the β-sheet on the interior of the particle core. Sidechains of basic residues in position to contact with the viral genome are shown and labeled. D The sequence of LY1 is shown for the ARM, jelly roll, and C-terminal regions. Basic residues of LY1 positioned to potentially contact the viral genome and shown in (C) are indicated with asterisks above the sequence along with the secondary structure elements.