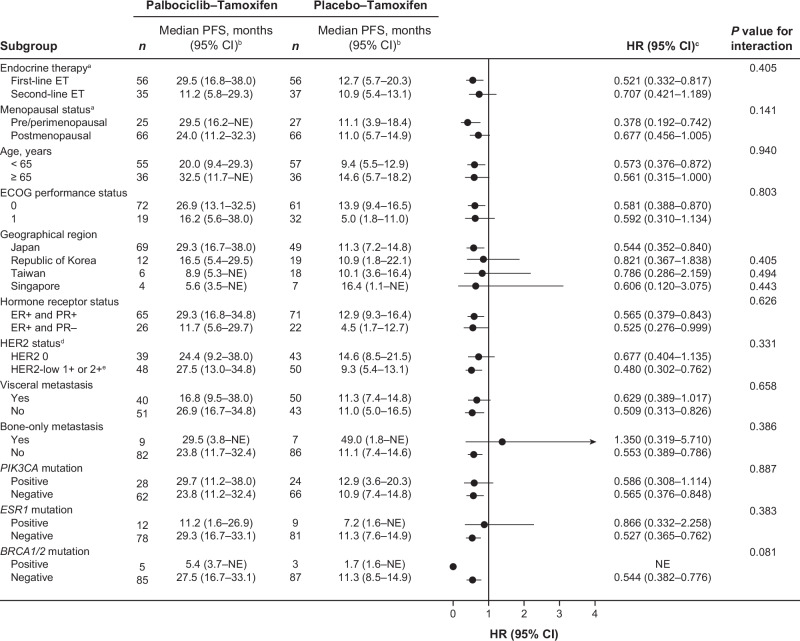
Fig. 3. Progression-free survival based on investigator assessment for all subgroups.
aBased on the registration system. bBrookmeyer and Crowley method. cHR and the corresponding 2-sided 95% CI for the palbociclib group relative to the placebo group were calculated by unstratified Cox proportional hazards model. dCategorized by IHC. Tests by IHC were not conducted in 4 patients in the Palbociclib-Tamoxifen group. eAll patients with HER2 2+ were negative by in situ hybridization. BRCA1/2 breast cancer 1 or 2 gene; CI confidence interval; ECOG Eastern Cooperative Oncology Group; ER estrogen receptor; ESR1 estrogen receptor 1 gene; ET endocrine therapy; HER2 human epidermal growth factor receptor 2-negative; HR hazard ratio; IHC immunohistochemistry; NE not estimable; PFS progression-free survival; PIK3CA phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha gene; PR progesterone receptor.