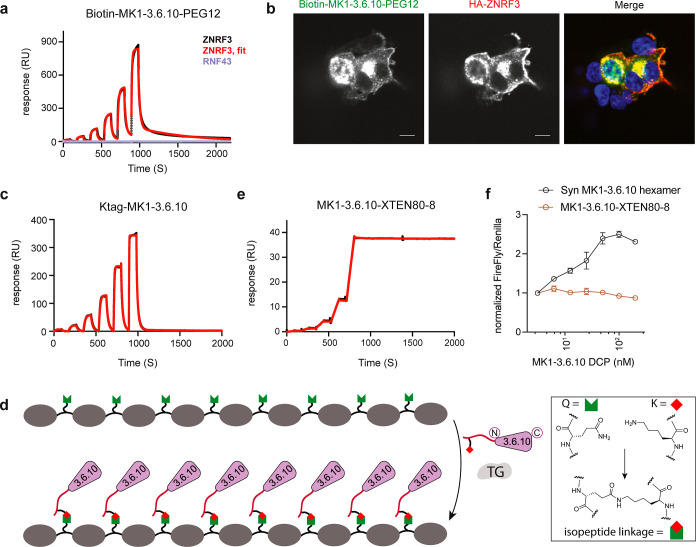
Figure 3.
Production and application of biotinylated MK1-3.6.10S (through STL) and octameric MK1-3.6.10 (through TG). (a) Biotinylated MK1-3.6.10S can be used as a binding assay reagent. Representative SPR sensorgram of biotin-MK1-3.6.10-PEG12 (immobilized on the sensor) binding to human ZNRF3 (black: raw data; red: fitted data) or RNF43 (purple). Biotin-MK1-3.6.10-PEG12 was generated through STL using recombinant MK1-3.6.10. (b) Biotin-MK1-3.6.10-PEG12 can be used to report cellular distribution of ZNRF3 in HEK293 cells transiently expressing HA-tagged ZNRF3. Biotin-MK1-3.6.10-PEG12 and the anti-HA antibody detected overexpressed ZNRF3 in the same cells, with a similar cellular distribution pattern. Scale bar: 10 μm. (c) Representative SPR sensorgram of Ktag-MK1-3.6.10 binding to human ZNRF3 (immobilized on the sensor). Black: raw data; red: fitted data. (d) Graphical representation of the enzymatic ligation of Ktag-MK1-3.6.10 to a Q-tag containing an XTEN polypeptide scaffold by transglutaminase. (e) Representative SPR sensorgram of octameric MK1-3.6.10 (MK1-3.6.10-XTEN80-8) binding to human ZNRF3 (immobilized on the sensor). Black: raw data; red: fitted data. MK1-3.6.10-XTEN generated through transglutaminase-mediated XTEN ligation demonstrated high affinity and extremely slow Koff (e) but failed to activate Wnt signaling in TOPbrite cells (f). Representative data from three independent runs are shown for all the assays.