Abstract
Background
The serum markers Krebs von den Lungen-6 (KL-6), surfactant protein A (SP-A), and surfactant protein D (SP-D) have been used for the diagnosis, differential diagnosis, and prognosis prediction of interstitial pneumonia. However, the significance of measuring the serum and bronchoalveolar lavage fluid (BALF) KL-6, SP-D, and SP-A levels in predicting the prognosis of chronic fibrosing interstitial pneumonia (CFIP), idiopathic pulmonary fibrosis, and idiopathic nonspecific interstitial pneumonia remains unclear. We aimed to clarify the significance of measuring the serum and BALF KL-6, SP-A, and SP-D levels in predicting the prognosis of patients with CFIP.
Methods
Among 173 patients who were diagnosed with CFIP between September 2008 and February 2021, 39 who underwent bronchoalveolar lavage were included in this study. Among these, patients experiencing an annual decrease in forced vital capacity (FVC) of ≥10% or those facing challenges in undergoing follow-up pulmonary function tests owing to significant deterioration in pulmonary function were categorized as the rapidly progress group. Conversely, individuals with an annual decrease in the FVC of <10% were classified into the slowly progress group. The serum and BALF KL-6, SP-D, and SP-A levels, as well as BALF/serum SP-D and SP-A ratios were compared between the two groups.
Results
Among the patients with CFIP, the BALF SP-D level (p=0.0111), BALF SP-A level (p<0.0010), BALF/serum SP-D ratio (p=0.0051), and BALF/serum SP-A ratio (p<0.0010) were significantly lower in the rapidly than in the slowly progress group (p<0.0010). The receiver operating characteristics analysis results demonstrated excellent performance for diagnosing patients with CFIP, with the BALF SP-D level (area under the curve [AUC], 0.7424), BALF SP-A level (AUC, 0.8842), BALF/serum SP-D ratio (AUC, 0.7673), and BALF/serum SP-A ratio (AUC, 0.8556). Moreover, the BALF SP-A level showed a notably superior CFIP diagnostic capability. Survival analysis using the Kaplan–Meier method revealed that patients with a BALF SP-A level of <1500 ng/mL and BALF/serum SP-A ratio of <15.0 had poor prognoses.
Conclusions
Our results suggest that BALF SP-A measurement may be useful for predicting the prognosis in patients with CFIP.
Keywords: Bronchoalveolar lavage fluid, Chronic fibrosing interstitial pneumonia, Prognosis, Surfactant protein A, Surfactant protein D
Background
Idiopathic interstitial pneumonia (IIP) encompasses diverse conditions with uncertain etiology, with idiopathic pulmonary fibrosis (IPF) representing a severe subtype of progressive lung fibrosis. Chronic fibrosing interstitial pneumonia (CFIP) is a broader category covering IPF and other fibrosing IIPs, emphasizing their fibrotic nature. While IIP necessitates pathological diagnosis, IPF is typically identifiable via high-resolution computed tomography (HRCT) [1]. In routine care, IIP diagnosis often avoids surgical biopsy due to its associated risks, with treatment administered accordingly. Hence, identifying prognostic predictors is meaningful in patients with CFIP diagnosed by HRCT or bronchoscopy (i.e., patients with confirmed IPF and others with CFIP), as well as those who are diagnosed using surgical lung biopsy, as this strategy will aid in decision-making regarding the treatment strategies.
Recently, the serum markers Krebs von den Lungen-6 (KL-6), surfactant protein A (SP-A), and surfactant protein D (SP-D) have been used for the diagnosis and differential diagnosis, as well as the prediction of prognosis in patients with interstitial pneumonia, including those with IPF [2–8]. The levels of these markers are measured in the serum and bronchoalveolar lavage fluid (BALF), although the patterns of increase in their values are not always consistent. The increase in the serum levels of KL-6, SP-D, and SP-A reflects their transfer from the alveolar epithelium into the blood vessels, whereas the increase in the BALF levels of these markers reflects their transfer from the alveolar epithelium to the air spaces. Therefore, investigating these relationships in detail is of interest in terms of understanding the pathological condition of interstitial pneumonia.
In this study, we aimed to clarify the significance of measuring the serum and BALF KL-6, SP-D, and SP-A levels in predicting the prognosis of patients with CFIP (IPF and suspected idiopathic nonspecific interstitial pneumonia).
Methods
Patients
Among 173 patients who were diagnosed with CFIP (including 135 with confirmed IPF) between September 2008 and February 2021 in our institution, 39 who underwent bronchoalveolar lavage (BAL) (including 24 with confirmed IPF) were included in this study. In this study, chronic fibrosing interstitial pneumonia (CFIP) includes progressive interstitial pneumonias with fibrosis, such as idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonia (NSIP). Cases suspected of collagen diseases or chronic hypersensitivity pneumonitis were excluded beforehand based on clinical symptoms of autoimmune diseases, autoantibody tests, the presence of other organ lesions, environmental history, and occupational history from medical records. Therefore, CHP or CTD-IP, which do not belong to IIPs, are not included. The other CFIP group includes cases without evident honeycombing on imaging, which are fNSIP cases, but the possibility of including IPF cases cannot be ruled out.
Diagnosis of interstitial pneumonia
A diagnosis of interstitial pneumonia was made by two radiologists and one pulmonologist based on chest CT findings at the first visit. Patients with confirmed IPF were defined as having a video-assisted thoracic surgery (VATS)-based diagnosis or presenting with honeycomb lung. Patients with predominant ground glass shadows in the bilateral lower lobes just below the pleura, reticular shadows, and traction bronchiectasis without an obvious honeycomb structure were defined as “patients with other CFIP.”
Investigated items
The following variables were investigated in the BAL group: age at the time of BAL; sex; VATS (present/absent); tissue type; smoking history; forced vital capacity (FVC) at the time of BAL; %FVC; serum albumin; and serum and BALF KL-6, SP-D, and SP-A levels. Additionally, the length of the observation period, the FVC, the %FVC, and whether or not treatment was performed after BAL were examined.
The following variables were investigated in the non-BAL group: age; sex; VATS (present/absent); tissue type; smoking history; forced vital capacity (FVC); %FVC; serum albumin; and serum KL-6, SP-D, and SP-A levels at the initial visit. Additionally, the length of the observation period, the FVC, the %FVC, and whether or not treatment was performed after the initial visit were examined.
Prognostic evaluation
Before conducting the prognostic analysis, survival analysis was performed in the group of patients with confirmed IPF and the other group, excluding those with confirmed IPF. The prognosis of CFIP was evaluated based on the results of this survival analysis.
Patients were divided into either the “rapidly progress” or “the slowly progress” group. The “rapidly progress” group consisted of patients with an annual decrease in the FVC of ≥10% after BAL and those for whom follow-up pulmonary function tests were difficult to perform due to the significant deterioration in their pulmonary function [9]. The “slowly progress” group consisted of patients with an annual decrease in the FVC of <10%. The serum and BALF levels of KL-6, SP-D, and SP-A, as well as their serum/BALF ratios were compared between the two groups.
Evaluation of the prognostic performance of each biomarker
Receiver operating characteristic (ROC) analysis was employed to evaluate the efficacy of biomarkers in distinguishing between the rapidly and slowly progress groups.
Survival analyses using the biomarkers
Based on the ROC analysis results, the biomarkers having superior progress performance were identified, and the cutoff values for the BALF levels and BALF/serum ratios were determined. The Kaplan–Meier method was used for the survival analyses.
Prognostic performance of each biomarker and survival analysis in patients with confirmed IPF and other CFIP
The prognostic performance of the biomarkers showing significant differences between the rapidly and slowly progress groups was assessed by ROC analysis in patients with confirmed IPF and other CFIP. ROC analysis followed by survival analysis was conducted to assess the diagnostic ability of each biomarker in the BALF and their BALF/serum ratios.
Statistical analyses
Nonparametric analyses were performed because the data did not show a normal distribution. The values are presented as medians with interquartile ranges. The Mann–Whitney U and Chi-squared test were used for between-group comparisons. The progress performance of each biomarker was assessed by ROC analysis. In addition, based on the results of the ROC analysis, the cutoff BAL values and BALF/serum ratios were determined for each biomarker; the survival analysis was performed using the Kaplan–Meier method. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using Excel Statistics (BellCurve for Excel version 3.21: Social Information Service Co., Ltd., Tokyo, Japan).
This study was approved by the Ethics Committee of National Hospital Organization Omuta National Hospital (Approval number: 29-14) and was conducted in accordance with the tenets of the Declaration of Helsinki.
Results
Characteristics of patients with CFIP
The patients’ characteristics are shown in Table 1.
Table 1.
Patients’ characteristics (n=173)
| Total (n=173) | BAL group (n=39) | Non-BAL group (n=134) | p-value | |
|---|---|---|---|---|
| Age (years) | 82 (74–88) | 74 (69–78) | 84 (78–90) | <0.001 |
| Sex (male/female) | 121/52 | 28/11 | 93/41 | 0.9296 |
| UIP/Others | 135 (VATS diagnosed patients: 4)/38 | 24 (VATS diagnosed patients: 4)/15 | 111/23 | 0.0091 |
|
Smoking history Present/Absent |
107/66 | 21/18 | 86/48 | 0.1286 |
| Survivor/Non-survivor | 86/87 | 18/21a | 68/66b | 0.7468 |
|
FVC (mL)c %FVC (%)c |
2350 (1585–2890) 78.85 (62.6-93.4) |
2180 (1510–2640) 72.2 (61.1–84.8) |
2400 (1620–2960) 82.3 (65.3–95.6) |
0.1650 0.0468 |
| Serum Alb (g/mL)c | 3.9 (3.5–4.2) | 4.1 (3.7-4.2) | 3.8 (3.5–4.1) | 0.0304 |
| Serum KL-6 (U/mL)c | 733 (435–1168) | 1130 (544–1695) | 686 (421–927) | <0.001 |
| Serum SP-D (ng/mL)c | 154.5 (88.5–305.3) | 262.0 (165.0–357.5) | 137.0 (79.0–240.5) | <0.001 |
| Serum SP-A (ng/mL)c | 56.5 (38.3–82.5) | 71.4 (45.1–113.2) | 50.6 (36.1–73.5) | 0.0034 |
| Antifibrotic drugs Present/Absent | 43/173 | 14/39 | 29/134 | 0.2429 |
|
Progression Good/Poor/unknownd |
52/38/83 | 19/20/0 | 33/18/83 | <0.001 |
Data are expressed as medians (ranges)
Alb albumin, BAL bronchoalveolar lavage, FVC forced vital capacity, KL-6 Krebs von den Lungen-6, SP-A surfactant protein A, SP-D surfactant protein D, UIP usual interstitial pneumonia, VATS video-assisted thoracic surgery
aIncluding four cases of death from other diseases and five cases of acute exacerbation
bIncluding 33 cases of death from other diseases and 21 cases of acute exacerbation
cat the time of BAL of BAL group and at the initial visit of Non-BAL group
dIn the non-BAL group, 83 patients did not have pulmonary function tests performed after the initial visit, so progression could not be assessed
Among 173 patients with CFIP, 39 had undergone BAL (BAL group). Concerning the type of CFIP, 135 patients had confirmed IPF, of whom four were diagnosed using VATS and underwent BAL, and 38 had CFIP other than confirmed IPF. The proportion of patients with CFIP other than confirmed IPF was significantly higher in the BAL group. Moreover, the age and %FVC at the initial visit were significantly lower, and the serum KL-6, SP-D, and SP-A levels were significantly higher in the BAL group than in the non-BAL group. Additionally, the BAL group displayed a significantly worse prognosis (Table 1).
The median age of patients with CFIP in the BAL group was 74 years, with no significant difference observed between the rapidly and slowly progress groups. The proportion of male patients was higher than that of female patients in the BAL group, with no significant difference observed between the rapidly and slowly progress groups. Out of a total of 39 CFIP patients, there were 24 patients with confirmed IPF, including four diagnosed via VATS, and 15 patients with CFIP other than confirmed IPF. No significant difference was observed in the number of patients with confirmed IPF and that of those with other CFIP between the rapidly and slowly progress groups. Moreover, no significant difference was observed between the rapidly and slowly progress groups in terms of history of smoking, with 21 and 18 patients being smokers and non-smokers, respectively, in the BAL group. The median period from the date of BAL to the date of last observation was 2.1 years, with a significantly shorter observation period in the rapidly progress group relative to that in the slowly progress group. There were 17 disease-specific death cases in both groups, with the rapidly progress group having a significantly higher number of deaths than the slowly progress group. The median FVC at the time of BAL was 2180 mL, with no significant difference observed between the rapidly and slowly progress groups. The median serum albumin, KL-6, SP-D, and SP-A levels at the time of BAL were 4.1 g/dL, 1130.0 U/mL, 262.0 ng/mL, and 71.4 ng/mL, respectively, with no significant differences observed between the rapidly and slowly progress groups. Concerning treatment, antifibrotic drugs were used in 16 patients in the BAL group, with no significant difference observed between the rapidly and slowly progress groups (Table 2).
Table 2.
Patients’ characteristics stratified according to progression (n=39)
| Total (n=39) | Rapidly progress group (n=20) | Slowly progress group (n=19) | p-value | |
|---|---|---|---|---|
| Age (years) | 74 (69–78) | 76 (70–82) | 74 (70–76) | 0.3676 |
| Sex (male/female) | 28/11 | 16 /4 | 12/7 | 0.4166 |
| UIP/Others | 24 (VATS diagnosed patients: 4)/15 | 13/7 | 11 (VATS diagnosed patients: 4)/8 | 0.8452 |
|
Smoking history Present/Absent |
21/18 | 11/9 | 10/9 | 1.0000 |
| Period from the time of BAL to the date of last observation (years) | 2.1 (0.8–4.2) | 1.2 (0.6–2.1) | 3.7 (1.9–5.6) | 0.0086 |
| Survivor/Non-survivor | 18/21 | 5/15a | 13/6b | 0.0020 |
|
FVC (mL) %FVC (%) at the time of BAL |
2180 (1510–2640) 72.2 (61.1–84.8) |
2050 (1555–2465) 69.7 (58.4–84.0) |
2500 (1500–2780) 78.5 (61.5–87.3) |
0.4313 0.3914 |
| Serum Alb at the time of BAL | 4.1 (3.7–4.2) | 3.9 (3.7–4.2) | 4.1 (3.8–4.3) | 0.6580 |
| Serum KL-6 at the time of BAL (U/mL) | 1130 (544–1695) | 1350 (785–1810) | 914 (485–1365) | 0.1643 |
| Serum SP-D at the time of BAL (ng/mL) | 262.0 (165.0–357.5) | 301.5 (175.5–439.3) | 209.0 (153.0–268.5) | 0.1440 |
| Serum SP-A at the time of BAL (ng/mL) | 71.4 (45.1–113.2) | 95.4 (58.7–111.1) | 57.8 (39.5–113.2) | 0.1562 |
|
Antifibrotic drugs Present/Absent |
16/23 | 10/10 | 6/13 | 0.3990 |
Data are expressed as medians (ranges)
Alb albumin, BAL bronchoalveolar lavage, FVC forced vital capacity, KL-6 Krebs von den Lungen-6, SP-A surfactant protein A, SP-D surfactant protein D, UIP usual interstitial pneumonia, VATS video-assisted thoracic surgery
aIncluding one case of death from other diseases and five cases of acute exacerbation
bIncluding three cases of death from other diseases and none of acute exacerbation
Comparison of each biomarker in the rapidly and slowly progress groups
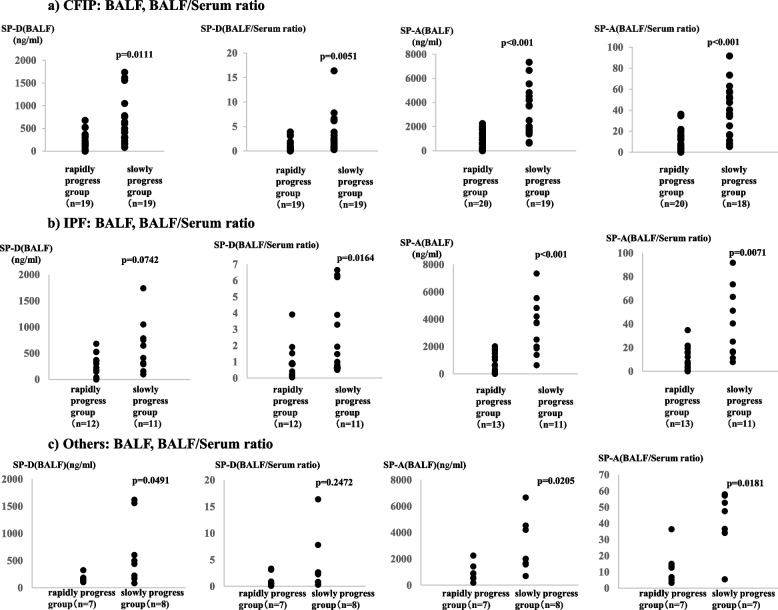
In CFIP patients, there was no significant difference in serum SP-D values between the rapidly and slowly progress groups, but BALF SP-D values and BALF/serum ratios were significantly higher in the slowly progress group (Fig. 1a). Similarly, there was no significant difference in serum SP-A values between the rapidly and slowly progress groups, but BALF SP-A values and BALF/serum SP-A ratio were significantly higher in the slowly progress group (Fig. 1a).
Fig. 1.
a CFIP: BALF, BALF/serum ratio. Comparison between the BALF SP-D and SP-A levels and the BALF/serum SP-D and SP-A ratios in the poor and good prognoses groups in patients with CFIP; b IPF: BALF, BALF/serum ratio. Comparison between the BALF SP-D and SP-A levels and the BALF/serum SP-D and SP-A ratios in the poor and good prognoses groups in patients with IPF; c Others: BALF, BALF/serum ratio. Comparison between the BALF SP-D and SP-A levels and the BALF/serum SP-D and SP-A ratios between the poor and good prognoses groups in patients with CFIP excluding those with confirmed IPF. BALF, bronchoalveolar lavage fluid; CFIP, chronic fibrosing interstitial pneumonia; IPF, idiopathic pulmonary fibrosis; SP-A, surfactant protein A; SP-D, surfactant protein D
In patients with confirmed IPF, there was no significant difference in serum and BALF SP-D levels between the rapidly and slowly progress groups, but the BALF/serum ratio was significantly higher in the slowly progress group (Fig. 1b). There was no significant difference in serum SP-A values between the rapidly and slowly progress groups, but BALF SP-A values and BALF/serum SP-A ratio were significantly higher in the slowly progress group (Fig. 1b).
In other CFIP patients, there were no significant differences in serum SP-D levels and BALF/serum SP-D ratio between the rapidly and slowly progress groups, but BALF SP-D levels were significantly higher in the slowly progress group (Fig. 1c). Similarly, there was no significant difference in serum SP-A values between the rapidly and slowly progress groups, but BALF SP-A values and BALF/serum SP-A ratio were significantly higher in the slowly progress group (Fig. 1c).
Prognostic performance of each biomarker
The results of the ROC analysis
The results of the ROC analysis revealed that the progress performance of the BALF SP-D level (area under the curve [AUC], 0.7424), BALF SP-A level (AUC, 0.8842), BALF/serum SP-D ratio (AUC, 0.7673), and BALF/serum SP-A ratio (AUC, 0.8556) was excellent, with that of the BALF SP-A level being notably superior (Table 3).
Table 3.
Receiver operating characteristics analysis
| AUC | Cutoff value | p-value | |
|---|---|---|---|
| CFIP | |||
| SP-D (BALF) | 0.7424 | 369 | 0.0029 |
| SP-D (BALF/serum ratio) | 0.7673 | 0.9251 | <0.001 |
| SP-A (BALF) | 0.8842 | 1500 | <0.001 |
| SP-A (BALF/serum ratio) | 0.8556 | 15.0 | <0.001 |
| IPF | |||
| SP-D (BALF) | 0.7197 | 300 | 0.0453 |
| SP-D (BALF/serum ratio) | 0.7955 | 1.0 | 0.0018 |
| SP-A (BALF) | 0.9021 | 1500 | <0.001 |
| SP-A (BALF/serum ratio) | 0.8252 | 17.0 | <0.001 |
| Others | |||
| SP-D (BALF) | 0.8036 | 325 | 0.0213 |
| SP-D (BALF/serum ratio) | 0.6786 | 0.80 | 0.2423 |
| SP-A (BALF) | 0.8571 | 1500 | <0.001 |
| SP-A (BALF/serum ratio) | 0.8571 | 15.0 | 0.0041 |
AUC area under the curve, BALF bronchoalveolar lavage fluid, CFIP chronic fibrosing interstitial pneumonia, IPF idiopathic interstitial pneumonia, SP-A surfactant protein A, SP-D surfactant protein D
Survival analysis using the BALF SP-D level, BALF SP-A level, BALF/serum SP-D ratio, and BALF/serum SP-A ratio
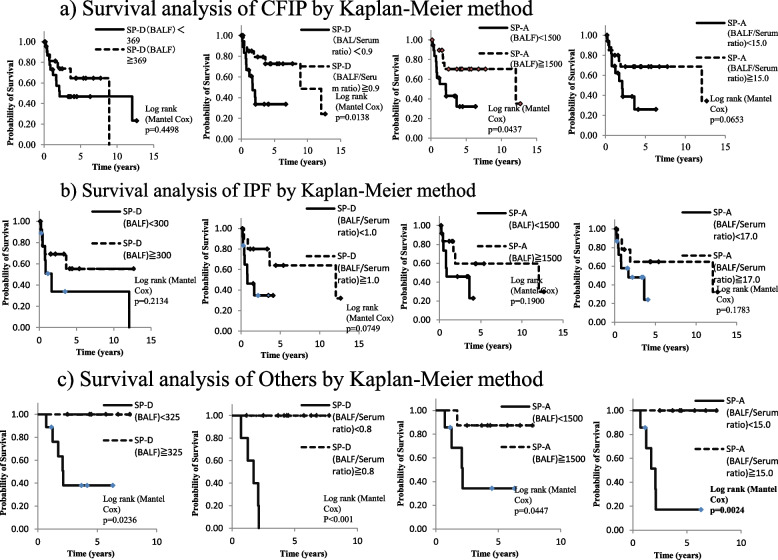
The cutoff BALF SP-D level, BALF SP-A level, BALF/serum SP-D ratio, and BALF/serum SP-A ratio, which showed excellent progress performance in the ROC analysis, were determined to be 369 ng/mL, 1500 ng/mL, 0.9, and 15.0, respectively. The survival analysis using these cutoff values revealed that patients with a BALF SP-A level of ≥1500 ng/mL and those with a BALF/serum SP-D ratio of ≥0.9 had a significantly better prognosis, and those with a BALF/serum SP-A ratio of ≥15.0 tended to have a better prognosis (p=0.0437, p=0.0138, and p=0.0653, respectively; Fig. 2a, Fig. 3.
Fig. 2.
Survival analyses of patients with (a) CFIP; (b) IPF; and (c) CFIP excluding those with confirmed IPF. The Kaplan–Meier method was used for the survival analyses. CFIP, chronic fibrosing interstitial pneumonia; IPF, idiopathic pulmonary fibrosis
Fig. 3.
a Survival analysis of CFIP by Kaplan-Meier method. b Survival analysis of IPF by Kaplan-Meier method. c Survival analysis of Others by Kaplan-Meier method
Progress performance of the BALF SP-D level, BALF SP-A level, BALF/serum SP-D ratio, and BALF/serum SP-A ratio in patients with confirmed IPF and other CFIP
The ROC analysis revealed that the progress performance of the BALF SP-D level (AUC, 0.7197), BALF SP-A level (AUC, 0.9021), BALF/serum SP-D ratio (0.7955), and BALF/serum SP-A ratio (AUC, 0.8252) was excellent in patients with confirmed IPF (Fig. 2b, Table 3); further, that of the BALF SP-D level (AUC, 0.8036), BALF SP-A level (AUC, 0.8571), BALF/serum SP-D ratio (0.6786), and BALF/serum SP-A ratio (AUC, 0.8571) was excellent in patients with other CFIP (Fig. 2c, Table 3). These results suggest that BALF SP-A measurement can be useful in predicting the progress in both patients with confirmed IPF and those with other CFIP.
Survival analysis using the BALF SP-A level and BALF/serum SP-A ratio in patients with confirmed IPF and other CFIP
Based on the ROC analysis of IPF, patients were divided into two groups according to the cutoff BALF SP-D level (300 ng/mL), BALF SP-A level (1500 ng/mL), BALF/serum SP-D ratio (1.0), and BALF/serum SP-A ratio (17.0). The results of the survival analysis revealed no significant difference between the two groups (Fig. 2b). For CFIP cases other than confirmed IPF, employing cutoff values of 325 ng/mL for the BALF SP-D level, 1500 ng/mL for the BALF SP-A level, 0.8 for the BALF/serum SP-D ratio, and 15.0 for the BALF/serum SP-A ratio, patients with BALF SP-D levels ≥325 ng/mL, BALP SP-A levels ≥1500 ng/mL, BALF/serum SP-D ratios ≥0.8, and BALF/serum SP-A ratio ≥15.0 exhibited better prognoses (p=0.0236, p=0.0447, p<0.001, and p=0.0024, respectively; Fig. 2c).
Discussion
The prognosis of IPF is poor, with a reported 5-year survival rate of 20–40% [10]. In CFIP, diagnostic imaging with HRCT or surgical biopsy/cryobiopsy is used for pathological diagnosis to differentiate between IPF and nonspecific interstitial pneumonia. Meanwhile, owing to the invasive nature of surgical biopsy/cryobiopsy and the advanced skills required for these procedures, pathological diagnosis is often avoided despite the need to make a differential diagnosis between IPF and idiopathic nonspecific interstitial pneumonia when HRCT does not show the typical usual interstitial pneumonia (UIP). Accordingly, the reported prognostic predictors of CFIP were mostly investigated in relation to IPF.
In CFIP cases, serial changes in the FVC are considered the best markers for monitoring disease progression [9]. In the present study, no significant differences were observed between the rapidly and slowly progress groups regarding FVC and %FVC at the time of BAL, suggesting that comparing these two groups is appropriate in evaluating the biomarker levels in BALF.
Prognosis is predicted based on the tests performed at the time of initial medical examination, rather than by evaluating progress through serial changes in prognostic markers, such as the FVC.
In clinical practice, it is meaningful to examine prognostic markers in patients other than those diagnosed with typical IPF by HRCT or those diagnosed with IPF by surgical biopsy and to identify prognostic markers in patients with CFIP. However, to date, no previous study has comprehensively examined BALF.
Prognostic serum biomarkers for CFIP have been reported in several studies, although there is insufficient evidence regarding their use in daily clinical practice.
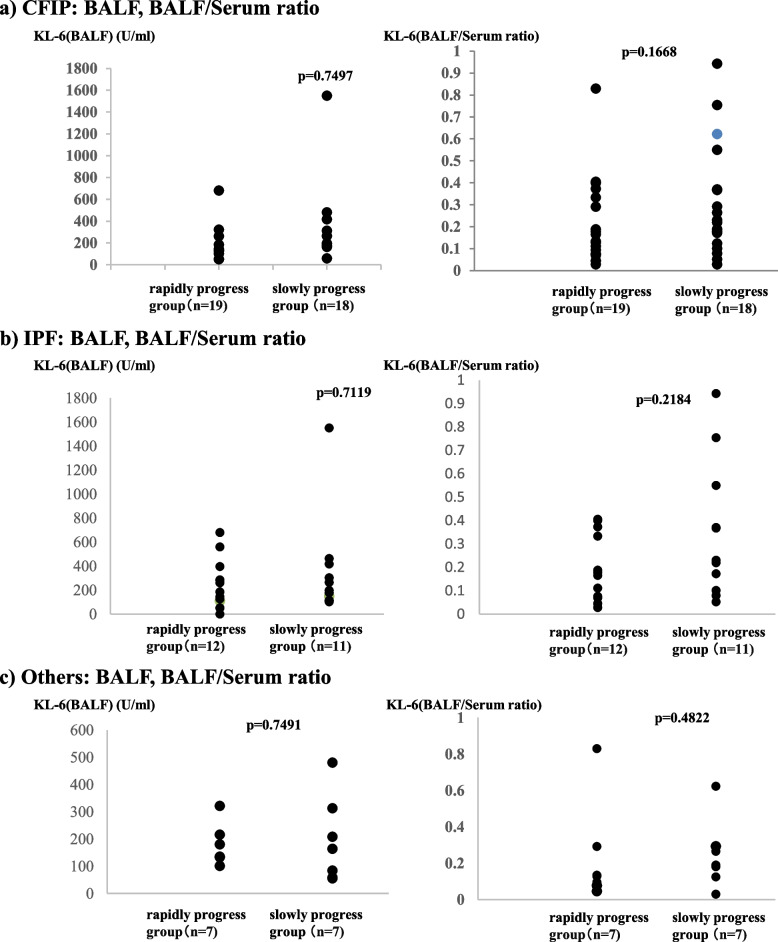
Concerning KL-6, serial changes in its serum levels are associated with prognosis in patients with IPF [2, 3]. Moreover, patients with a serum KL-6 level of ≥1000 U/mL [4–6] have poor prognoses. KL-6 is a glycoprotein primarily expressed on the extracellular membrane surface of type II alveolar epithelial cells, and it may be involved in the promotion of migration, proliferation, and survival of lung fibroblasts [11, 12]. In CFIP, inflammation and fibrosis occur in lung tissue, causing damage to alveolar epithelial cells. This damage leads to the secretion of KL-6 from the alveolar epithelial cells. Additionally, KL-6 may be overexpressed in type II alveolar epithelial cells during the repair process of damaged alveolar epithelial cells. In this study, no correlation was found between the concentration of KL-6 in BALF and disease progression. However, the rapidly progress group, although not showing a significant difference, exhibited higher values than the slowly progress group, with a median serum KL-6 level of ≥1000 U/mL. This is consistent with previous reports and may reflect damage to alveolar epithelial cells. Because KL-6 is a glycoprotein expressed on the surface of type II alveolar epithelial cells, it is speculated that in the favorable prognosis group, the repair mechanism progresses efficiently, reducing the extent of damage, thus preventing excessive secretion of KL-6.
On the other hand, in the slowly progress group, it is thought that surfactant is actively produced by type II alveolar epithelial cells, repairing the damaged alveolar epithelium and maintaining alveolar function through excessive secretion.
Regarding SP-D, patients with serum SP-D levels of ≥250 ng/mL [7] have been reported to have a poor prognosis, and in our study, the median SP-D level in the rapid progression group was also ≥250 ng/mL. In our study, there was a significant difference in BALF SP-D levels between the rapidly progress and slowly progress groups of CFIP. However, survival analysis did not show a significant difference between BALF SP-D ≥ 369 ng/mL and SP-D < 369 ng/mL. Nevertheless, the survival curve of BALF SP-D ≥ 369 ng/mL consistently showed higher survival rates compared to SP-D < 369 ng/mL, suggesting that a larger sample size might yield a significant difference. The BALF/serum ratio of SP-D was significantly lower in the rapidly progress group. This finding suggests that SP-D, being more hydrophilic compared to SP-A, tends to migrate into the serum [13].
Patients with serum SP-A levels of ≥80 ng/mL [8] have been reported to have a poor prognosis, and in our study, the median level in the rapidly progress group was also ≥80 ng/mL. There are few prior studies examining BALF. McCormack et al. reported that in IPF, patients who died within 2 years had lower BALF SP-A/PL compared to survivors, and patients with low BALF SP-A/PL levels had a poorer prognosis according to survival curves [14]. Although this study mainly focused on CFIP patients, an analysis limited to confirmed cases of IPF revealed that BALF SP-A levels had the highest prognostic value, consistent with previous reports. Furthermore, Nishikiori et al. reported no difference in BALF SP-A levels between IPF patients and healthy individuals [13]. Although we did not examine BALF SP-A levels in healthy individuals in our study, it is plausible that the lack of significant findings in Nishikiori et al.'s study could be attributed to the inclusion of both good and poor prognosis cases among the IPF patients they investigated.
Our findings are of high clinical significance, as similar results were obtained in patients with CFIP excluding those with HRCT- or pathologically-diagnosed UIP.
We previously reported that, in patients with IIP and UIP diagnosed by surgical biopsy, the SP-A expression in the lesion tissue significantly decreased in patients with a poor prognosis [13]. Takezaki et al. performed a genomic analysis of families with familial IPF and reported that mutations in the gene encoding SFTPA1, one of the constituent molecules of SP-A, cause hyposecretion of SFTPA1 from type II alveolar epithelial cells, thereby increasing the sensitivity of type II alveolar epithelial cells to necroptosis and possibly leading to pulmonary fibrosis [14]. This suggests that patients with decreased BALF SP-A levels may have had increased necroptosis of type II alveolar epithelial cells, which may have led to the progression of pulmonary fibrosis, resulting in their poor prognosis. Moreover, SP-A has also been reported to induce a natural immune effect [15]; thus, it is possible that the decrease in the production of SP-A in type II alveolar epithelial cells may have concurrently caused pulmonary infection and induced acute exacerbation and worsened the prognosis.
The present study had some limitations. First, the sample size was small as BAL was not performed in all patients with CFIP. This decision stemmed from the established diagnostic criteria for typical IPF, where imaging findings often suffice, making BAL unnecessary. In addition, BAL was performed in selected patients potentially exhibiting higher activity levels relative to others in the overall population of patients with CFIP. Second, this study was conducted at a single center. Thus, future studies with a larger sample size are needed. Third, it was not possible to pathologically diagnose all patients for whom a definitive diagnosis of UIP could not be made using HRCT. Despite these limitations, we believe that our findings are significant as they suggest the potential usefulness of BAL SP-A measurement in predicting the prognosis for patients with CFIP given the large number of cases, in which surgical biopsy is not feasible in clinical practice.
Conclusions
Our findings indicate the potential utility of BALF SP-A measurement as a prognostic marker in CFIP cases. Future multicenter studies may further validate its usefulness, facilitating treatment decisions without invasive procedures like surgical biopsy or cryobiopsy for CFIP.
Acknowledgements
Not applicable.
Abbreviations
- AUC
Area under the curve
- BAL
Bronchoalveolar lavage
- BALF
Bronchoalveolar lavage fluid
- CFIP
Chronic fibrosing interstitial pneumonia
- CT
Computed tomography
- FVC
Forced vital capacity
- HRCT
High-resolution computed tomography
- IIP
Idiopathic interstitial pneumonia
- IPF
Idiopathic pulmonary fibrosis
- KL-6
Krebs von den Lungen-6
- ROC
Receiver operating characteristic
- SP-A
Surfactant protein A
- SP-D
Surfactant protein D
- UIP
Usual interstitial pneumonia
- VATS
Video-assisted thoracic surgery
Authors’ contributions
KW assisted with the study design and data interpretation, had full access to the study data, assumes responsibility for the data integrity and accuracy of the analysis, and drafted the manuscript. NN, HK, MH, SA, NN, RK, IF, MT, KK, TA, SM, KM, JO, MI, MK, and HY assisted with the study design and data interpretation, and edited the initial manuscript draft. All authors reviewed the manuscript.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study was conducted after being approved by the ethics committee of National Hospital Organization Omuta National Hospital (Approval No.: 29-14). Informed consent was obtained from all subjects and their legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205:e18–47. 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Y, Luo Q, Han Q, Huang J, Ou Y, Chen M, et al. Sequential changes of serum KL-6 predict the progression of interstitial lung disease. J Thorac Dis. 2018;10:4705–14. 10.21037/jtd.2018.07.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakamatsu K, Nagata N, Kumazoe H, Oda K, Ishimoto H, Yoshimi M, et al. Prognostic value of serial serum KL-6 measurements in patients with idiopathic pulmonary fibrosis. Respir Investig. 2017;55:16–23. 10.1016/j.resinv.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 4.Hamai K, Iwamoto H, Ishikawa N, Horimasu Y, Masuda T, Miyamoto S, et al. Comparative study of circulating MMP-7, CCL18, KL-6, SP-A, and SP-D as disease markers of idiopathic pulmonary fibrosis. Dis Markers. 2016;2016:4759040. 10.1155/2016/4759040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology. 2006;11:164–8. 10.1111/j.1440-1843.2006.00834.x [DOI] [PubMed] [Google Scholar]
- 6.Satoh H, Kurishima K, Ishikawa H, Ohtsuka M. Increased levels of KL-6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med. 2006;260:429–34. 10.1111/j.1365-2796.2006.01704.x [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H, Shiratori M, Kanai A, Chiba H, Kuroki Y, Abe S. Monitoring markers of disease activity for interstitial lung diseases with serum surfactant proteins A and D. Respirology. 2006;11(Suppl):S51–4. [DOI] [PubMed] [Google Scholar]
- 8.Song JW, Do KH, Jang SJ, Colby TV, Han S, Kim DS. Blood biomarkers MMP-7 and SP-A: predictors of outcome in idiopathic pulmonary fibrosis. Chest. 2013;143:1422–9. 10.1378/chest.11-2735 [DOI] [PubMed] [Google Scholar]
- 9.Kim DS, Collard HR, King TE Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–92. 10.1513/pats.200601-005TK [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. 2011;184:1382–9. 10.1164/rccm.201105-0840OC [DOI] [PubMed] [Google Scholar]
- 11.McCormack FX, King TE Jr, Bucher BL, Nielsen L, Mason RJ. Surfactant protein A predicts survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1995;152:751–9. 10.1164/ajrccm.152.2.7633738 [DOI] [PubMed] [Google Scholar]
- 12.Nishikiori H, Chiba H, Ariki S, Kuronuma K, Otsuka M, Shiratori M, et al. Distinct compartmentalization of SP-A and SP-D in the vasculature and lungs of patients with idiopathic pulmonary fibrosis. BMC Pulm Med. 2014;14:196. 10.1186/1471-2466-14-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagata N, Kitasato Y, Wakamatsu K, Kawabata M, Fukushima K, Kajiki A, et al. Prognostic value of immunohistochemical surfactant protein A expression in regenerative/hyperplastic alveolar epithelial cells in idiopathic interstitial pneumonias. Diagn Pathol. 2011;6:25. 10.1186/1746-1596-6-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takezaki A, Tsukumo SI, Setoguchi Y, Ledford JG, Goto H, Hosomichi K, et al. A homozygous SFTPA1 mutation drives necroptosis of type II alveolar epithelial cells in patients with idiopathic pulmonary fibrosis. J Exp Med. 2019;216:2724–35. 10.1084/jem.20182351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campo I, Zorzetto M, Bonella F. Facts and promises on lung biomarkers in interstitial lung diseases. Expert Rev Respir Med. 2015;9:437–57. 10.1586/17476348.2015.1062367 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.