Abstract
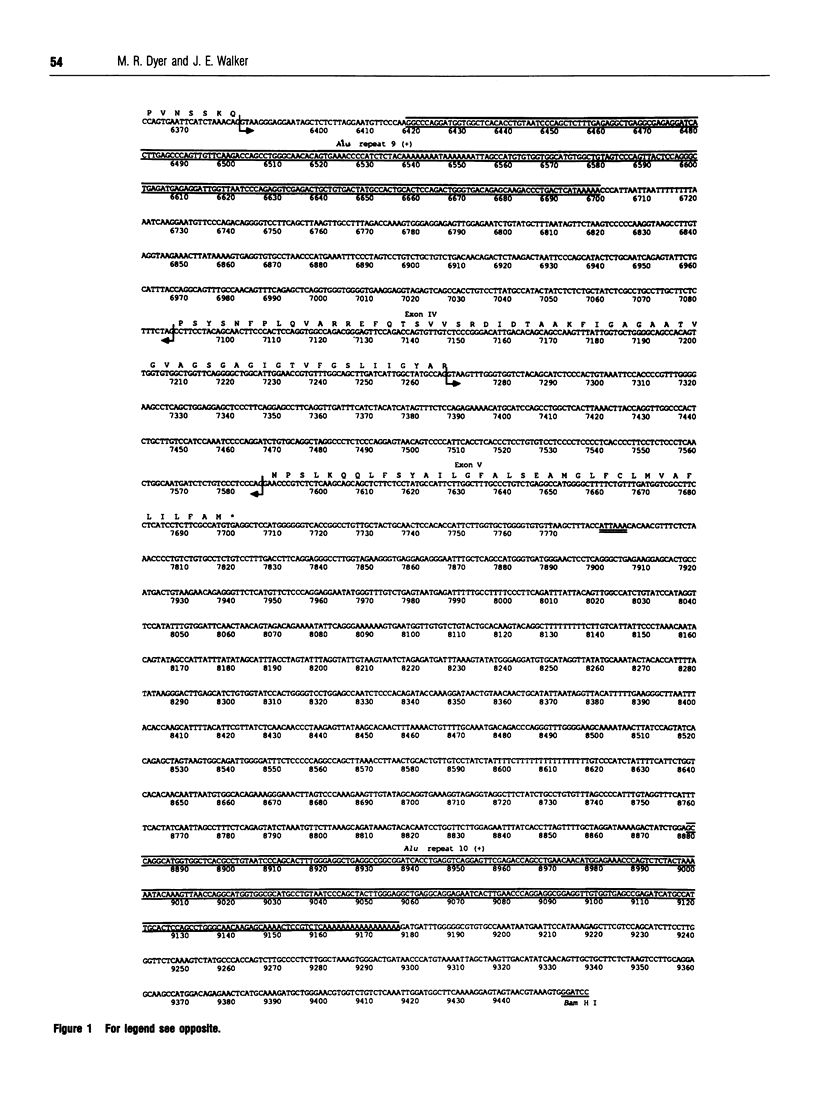
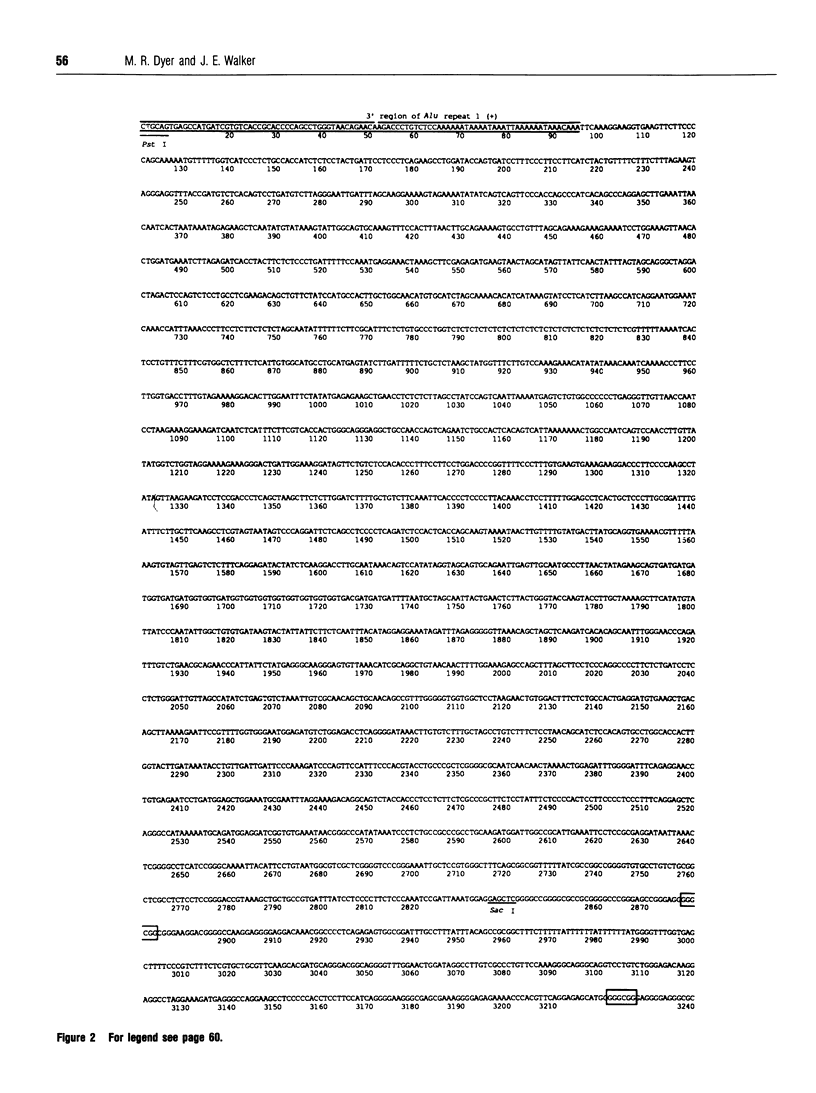
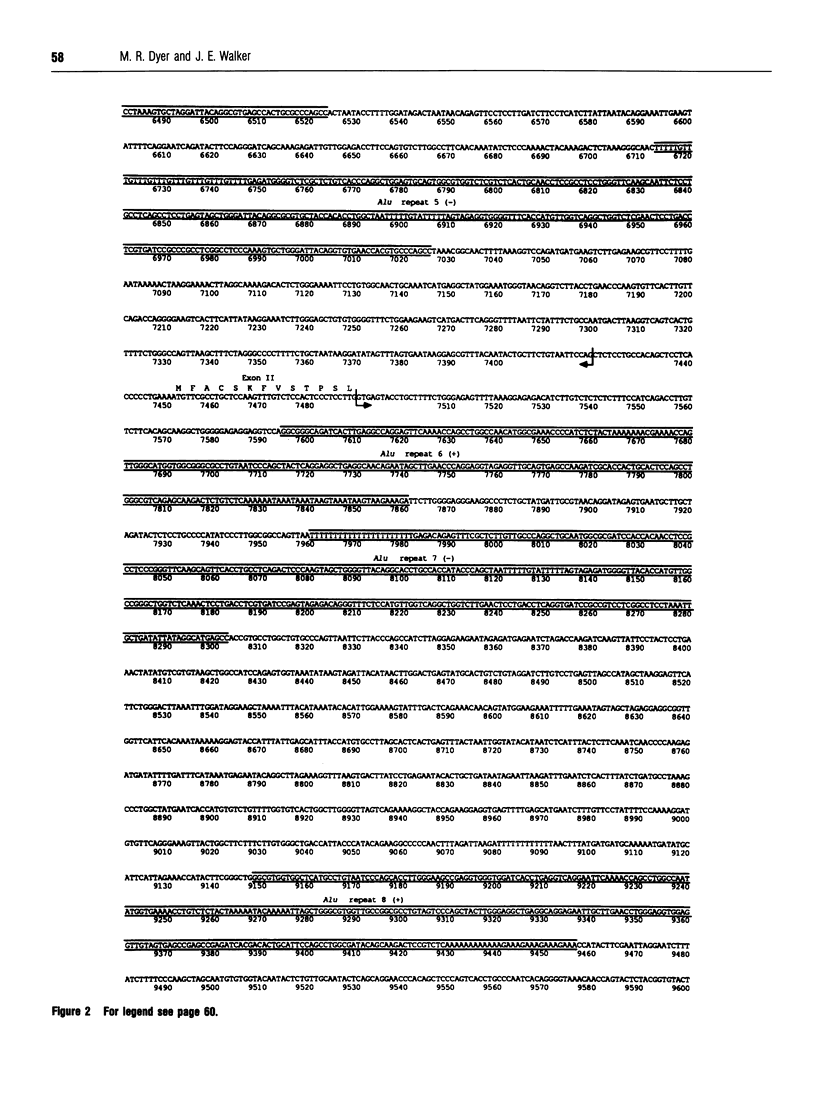
Subunit c is an intrinsic membrane component of ATP synthase, and in mammals it is encoded by two expressed nuclear genes, P1 and P2. Both genes encode the same mature c subunit, but the mitochondrial import pre-sequences in the precursors of subunit c are different. The DNA sequences of the human P1 and P2 genes are described. They occupy about 3.0 and 10.9 kb respectively of the human genome, and both genes are split into five exons. The human genome also contains about 14 related spliced pseudogenes, and the sequence of one such pseudogene related to P2 is described. Sequences flanking the 5' ends of the human P1 and P2 coding sequences each contain a CpG-rich island. Potential promoter elements (TATA and CCAAT boxes) are present in the 5' sequences of the P1 gene, but not that of P2, although there is no direct experimental evidence to show the involvement of these sequences in transcription of the genes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachman N. J., Lomax M. I., Grossman L. I. Two bovine genes for cytochrome c oxidase subunit IV: a processed pseudogene and an expressed gene. Gene. 1987;55(2-3):219–229. doi: 10.1016/0378-1119(87)90282-4. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berget S. M. Are U4 small nuclear ribonucleoproteins involved in polyadenylation? Nature. 1984 May 10;309(5964):179–182. doi: 10.1038/309179a0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Blake C. C. Exons encode protein functional units. Nature. 1979 Feb 22;277(5698):598–598. doi: 10.1038/277598a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cozens A. L., Runswick M. J., Walker J. E. DNA sequences of two expressed nuclear genes for human mitochondrial ADP/ATP translocase. J Mol Biol. 1989 Mar 20;206(2):261–280. doi: 10.1016/0022-2836(89)90477-4. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Dyer M. R., Gay N. J., Walker J. E. DNA sequences of a bovine gene and of two related pseudogenes for the proteolipid subunit of mitochondrial ATP synthase. Biochem J. 1989 May 15;260(1):249–258. doi: 10.1042/bj2600249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell L. B., Nagley P. Human liver cDNA clones encoding proteolipid subunit 9 of the mitochondrial ATPase complex. Biochem Biophys Res Commun. 1987 May 14;144(3):1257–1264. doi: 10.1016/0006-291x(87)91446-x. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Deininger P. L., Bankier A., Barrell B. Homologous upstream sequences near Epstein-Barr virus promoters. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1565–1569. doi: 10.1073/pnas.80.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley I. M., Walker J. E., Martinus R. D., Jolly R. D., Kirkland K. B., Shaw G. J., Palmer D. N. The sequence of the major protein stored in ovine ceroid lipofuscinosis is identical with that of the dicyclohexylcarbodiimide-reactive proteolipid of mitochondrial ATP synthase. Biochem J. 1990 Jun 15;268(3):751–758. doi: 10.1042/bj2680751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A., Huck S., Ghanem N., Lefranc M. P., Rabbitts T. H. New subgroups in the human T cell rearranging V gamma gene locus. EMBO J. 1987 Jul;6(7):1945–1950. doi: 10.1002/j.1460-2075.1987.tb02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner R. M. Mapping the gene for juvenile onset neuronal ceroid lipofuscinosis to chromosome 16 by linkage analysis. Am J Med Genet. 1992 Feb 15;42(4):539–541. doi: 10.1002/ajmg.1320420423. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Two genes encoding the bovine mitochondrial ATP synthase proteolipid specify precursors with different import sequences and are expressed in a tissue-specific manner. EMBO J. 1985 Dec 16;4(13A):3519–3524. doi: 10.1002/j.1460-2075.1985.tb04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Jackl G., Sebald W. Identification of two products of mitochondrial protein synthesis associated with mitochondrial adenosine triphosphatase from Neurospora crassa. Eur J Biochem. 1975 May;54(1):97–106. doi: 10.1111/j.1432-1033.1975.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Andersson M., Lodish H. F. Structure and organization of the murine band 3 gene. J Biol Chem. 1987 Jun 15;262(17):8035–8040. [PubMed] [Google Scholar]
- LeFranc M. P., Forster A., Baer R., Stinson M. A., Rabbitts T. H. Diversity and rearrangement of the human T cell rearranging gamma genes: nine germ-line variable genes belonging to two subgroups. Cell. 1986 Apr 25;45(2):237–246. doi: 10.1016/0092-8674(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: partial sequence of a mitochondrial ATPase gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Jan;76(1):131–135. doi: 10.1073/pnas.76.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini G., Toniolo D., Vulliamy T., Luzzatto L., Dono R., Viglietto G., Paonessa G., D'Urso M., Persico M. G. Structural analysis of the X-linked gene encoding human glucose 6-phosphate dehydrogenase. EMBO J. 1986 Aug;5(8):1849–1855. doi: 10.1002/j.1460-2075.1986.tb04436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey J. R., Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987 Apr 2;326(6112):501–505. doi: 10.1038/326501a0. [DOI] [PubMed] [Google Scholar]
- Medd S. M., Walker J. E., Jolly R. D. Characterization of the expressed genes for subunit c of mitochondrial ATP synthase in sheep with ceroid lipofuscinosis. Biochem J. 1993 Jul 1;293(Pt 1):65–73. doi: 10.1042/bj2930065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R. Structure-independent nucleotide sequence analysis. Proc Natl Acad Sci U S A. 1979 May;76(5):2232–2235. doi: 10.1073/pnas.76.5.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naora H., Deacon N. J. Relationship between the total size of exons and introns in protein-coding genes of higher eukaryotes. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6196–6200. doi: 10.1073/pnas.79.20.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4851–4855. doi: 10.1073/pnas.81.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Yohda M., Ishizuka M., Hirata H., Hamamoto T., Otawara-Hamamoto Y., Matsuda K., Kagawa Y. Sequence and over-expression of subunits of adenosine triphosphate synthase in thermophilic bacterium PS3. Biochim Biophys Acta. 1988 Mar 30;933(1):141–155. doi: 10.1016/0005-2728(88)90064-3. [DOI] [PubMed] [Google Scholar]
- Palmer D. N., Fearnley I. M., Walker J. E., Hall N. A., Lake B. D., Wolfe L. S., Haltia M., Martinus R. D., Jolly R. D. Mitochondrial ATP synthase subunit c storage in the ceroid-lipofuscinoses (Batten disease). Am J Med Genet. 1992 Feb 15;42(4):561–567. doi: 10.1002/ajmg.1320420428. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rinehart F. P., Ritch T. G., Deininger P. L., Schmid C. W. Renaturation rate studies of a single family of interspersed repeated sequences in human deoxyribonucleic acid. Biochemistry. 1981 May 26;20(11):3003–3010. doi: 10.1021/bi00514a003. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G., Imam G., Küntzel H. Mitochondrial ATPase complex of Aspergillus nidulans and the dicyclohexylcarbodiimide-binding protein. Eur J Biochem. 1979 Jul;97(2):565–571. doi: 10.1111/j.1432-1033.1979.tb13145.x. [DOI] [PubMed] [Google Scholar]
- Ullu E., Tschudi C. Alu sequences are processed 7SL RNA genes. Nature. 1984 Nov 8;312(5990):171–172. doi: 10.1038/312171a0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Gay N. J., Powell S. J., Kostina M., Dyer M. R. ATP synthase from bovine mitochondria: sequences of imported precursors of oligomycin sensitivity conferral protein, factor 6, and adenosinetriphosphatase inhibitor protein. Biochemistry. 1987 Dec 29;26(26):8613–8619. doi: 10.1021/bi00400a018. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Lutter R., Dupuis A., Runswick M. J. Identification of the subunits of F1F0-ATPase from bovine heart mitochondria. Biochemistry. 1991 Jun 4;30(22):5369–5378. doi: 10.1021/bi00236a007. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]