Abstract
Natural Killer (NK) cells are innate immune cells that play a pivotal role as first line defenders in the anti-tumor response. To prevent tumor development, NK cells are searching for abnormal cells within the body and appear to be key players in immunosurveillance. Upon recognition of abnormal cells, NK cells will become activated to destroy them. In order to fulfill their anti-tumoral function, they rely on the secretion of lytic granules, expression of death receptors and production of cytokines. Additionally, NK cells interact with other cells in the tumor microenvironment. In this review, we will first focus on NK cells’ activation and cytotoxicity mechanisms as well as NK cells behavior during serial killing. Lastly, we will review NK cells’ crosstalk with the other immune cells present in the tumor microenvironment.
Subject terms: Innate lymphoid cells, Immunosurveillance, Cell death and immune response
Facts
NK cells are first line defenders in cancer, by natural cytotoxicity and by mediating ADCC making them crucial components for cancer immunosurveillance.
NK cells have at their disposal several weapons in their arsenal such as cytotoxic molecules, cytokines, or death ligands, to destroy cancer cells.
Interaction between NK cells and innate or adaptative immune cells in the tumor microenvironment are various and complex, contributing to shape the anti-tumor response.
Open questions
How fundamental knowledge learnt in pre-clinical studies can help improving NK cell-based therapy?
To which extent ex vivo expanded NK cells used in therapy interact with immune cells in the tumor microenvironment?
There are multiple NK cell subsets, but their specific in fighting cancer are unknown.
Introduction
Natural Killer (NK) cells were first described in mice by Rolf Kiessling, Eva Klein and Hans Wigzell in the 1970s as “naturally occurring killer lymphocytes” able to destroy murine leukemia cells [1, 2]. Since then, NK cells have been thoroughly studied and relatively well described as part of the innate lymphoid cell (ILC) family.
NK cells are first-line defenders of the immune response against infectious diseases [3] and cancer [4] but they are also important in autoimmune [5, 6] and metabolic diseases [7]. They provide rapid responses because they do not require prior priming. They recognize target cells using germline-encoded surface receptors able to recognize activating and inhibitory signals. If more activating receptors are engaged, NK cells engage in two different pathways: directly kill the target cell or shape the tumor microenvironment through cytokine production and direct cell-cell interactions.
NK cells are defined as CD56+; CD3- and can be overall classified into two distinct subsets: CD56bright CD16- and CD56dim CD16+. Several reports suggested that CD56bright NK cells can differentiate into CD56dim subset [8, 9]. The first subset is thought to be a not fully mature group of NK cells which account for 5-10% of peripheral blood NK cells and can secrete large amounts of cytokines [10]. The second one is mature, produces lower cytokines and exerts strong antibody-dependent and independent cytotoxicity [11]. Moreover, less mature CD56dim NK cells can strongly respond to several cytokines including IL-12, IL-15 and IL-18, whereas more mature CD56dim NK cells lose this ability but increase their cytotoxic function [12]. Despite the above-mentioned classification, stating that CD56bright NK cells are mainly cytokines producers, it is worth mentioning that CD56bright NK cells can exert strong cytotoxic response in vitro and in vivo against tumor cells after IL-15 priming [13].
Two models exist regarding NK cell development in tissues. First, NK cells develop within bone marrow or secondary lymphoid organs. CD56bright and CD56dim NK cells migrate from secondary lymphoid organs or bone marrow respectively to peripheral organs, in which tissue-specific differentiation occurs. The second model proposes that NK cells migrate from bone marrow in an early development state to tissues, where they mature. This model is based on the fact that NK cell precursors have been found in several peripheral tissues such as the guts, uterus and liver [12]. Nevertheless, it is established that the compartment in which NK cells migrate drives their maturation to grant them tissue-specific phenotype [14, 15].
NK cells form a very diversified cell population that are found in most tissues. Indeed, NK cells reside in high numbers in the peripheral blood, in the lungs, uterus, liver and more rarely in lymph nodes and tonsils. For example, NK cells can represent up to 50% of the lymphocytes in the uterus [12]. Moreover, CD56bright NK cells are more frequent in the guts, tonsils, lymph nodes and the skin, whereas in the liver, the lungs and the spleen NK cells are mainly CD56dim [12].
T-bet and Eomes have long been reported as major drivers of NK cells development in mice [16]. In humans, T-bet and Eomes knock-out in NK cells reduce IFNγ, perforin (Prf) and granzyme B (GrzB) production as well as proliferation, thus impairing NK cells anti-tumor function in tumor-bearing NSG mice model [17]. Another transcription factor, IRF2, has recently been implicated in human NK cell proliferation and functional maturation [18]. Thus, NK cell’s development relies on several transcription factors, conferring them potent cytotoxic function.
In this review, we will focus on NK cell’s role in fighting cancer, reviewing knowledge from both human and mice studies. A summary of the difference between human and mice NK cell has been published [19]. First, we will focus on NK cells’ cytotoxic arsenal and then, on NK cell’s communication with other immune cells in the tumor microenvironment (TME).
NK cell cytotoxic Arsenal
Immunosurveillance and importance in cancer
NK cells are known to be one of the main drivers of cancer immunosurveillance. NK cells are patrolling within the body hunting for abnormal cells, such as transformed cells, preventing tumor development [20]. Moreover, a link between the cytotoxic capability of peripheral blood lymphocytes and cancer occurrence in a Japanese cohort has been unveiled [21]. It is most likely that NK cells account for a significant part of the cytotoxic PBMCs, supporting their importance in immunosurveillance [21]. In this line, NK cells anti-tumor activity is important for tumor control. Indeed, NK cell exhaustion and cytokine secretion defect have been linked to impaired survival in patients with newly diagnosed multiple myeloma [22], and low NK cell count in the peripheral blood is associated with impaired survival in follicular lymphoma patients [23]. Additionally, tumor-infiltrating NK cells are linked to better survival in several solid cancers including head and neck cancer, breast cancer, lung cancer and others [24]. NK cells are also known to target circulating tumor cells [25] that underwent epithelial to mesenchymal transition, which is part of the metastatic process [26]. Indeed, circulating tumor cells express ligands such as E-cadherin and CDMA1 [27], which are recognized by NK cells and induce their cytotoxicity. Furthermore, in a mouse model, NK cell depletion led to an increase in the number of pulmonary metastases whereas T cell depletion did not impact the number but the area of the metastatic foci. This paper highlights the ability of NK cells to “clear” circulating cancer cells and their role in immunosurveillance [28]. Although numerous works place NK cell as one of the main protagonists in the fight against cancer, it is important to balance this by emphasizing that NK cell’s functions are greatly impaired in the TME. Immunosuppressive cells, such as M2 macrophages or regulatory T cells, soluble factors, such as IL-10 or TGF-β, and membrane-bound ligands, such as immune checkpoint inhibitors including PD-1 and TIM-3, are all factors able to inhibit NK cells activity [29]. Cell-cell interaction modulating NK cell activity will be specifically discussed later in this review. Moreover, it was recently shown in a mice model that NK cell loss of effector functions happens rapidly after NK cell entry in the tumor, revealing a probable short-term action of NK cells after tumor engagement [30].
Immune synapse formation: stick close to your enemy
NK cells’ cytotoxic activity is mainly mediated by the release of lytic granules which contain cytotoxic molecules. This tightly regulated mechanism occurs through sequential steps [31]. NK cells interact with target cells through several receptors. Some of them are activating, others inhibitory. If activating signals are superior to inhibitory, the immune synapse (IS) is formed. The IS formation is initialized by integrins ligation expressed by NK cells such as LFA-1 to its cognate ligands ICAM-1/2 [32–34]. This interaction will stabilize the IS thanks to a firm adhesion to the target cell [35] and also induce an actin reorganization within the NK cells through clustering of activating receptors. Interestingly, CD56, the major surface recognition marker of NK cells also plays an adhesion role [36]. More recently, CD56 engagement with a target cell was showed to be necessary for IS formation, intracellular target phosphorylation, and degranulation, as CD56 KO NK cell line failed to do so [37]. Upon engagement with ICAM-1/2, LFA-1 allows lytic granule guidance to the IS and in addition induces strong degranulation through mechanosensing [38]. Intracellular signals from clustered activating receptors induce MTOC polarization which drives scattered lytic granules within NK cells cytosol to converge to the IS by allowing them to travel on microtubules [39]. Once near the IS, lytic granules will then move onto the actin filaments until reaching the cell membrane where their content will be locally released in the synaptic cleft onto the target cell through exocytosis [40]. Finally, IS maintenance was shown to be an active process, since sustained signaling from engaged activating receptors maintains it during the killing event [41].
NK cells activating receptors
NK cell cytotoxicity is governed by a plethora of activating and inhibitory receptors expressed at their membrane. By interacting with target cell ligands, a balance between activating and inhibiting signals will be integrated within the NK cell and decide the fate of the target cell: live or die [42].
Activating receptors are associated with different intracellular chains that transduce signals. For instance, Natural Cytotoxic Receptors (NCR), NKG2C, an activating receptor belonging to the NKG2 family, and CD16a interact with immunoreceptor tyrosine-based activation motif (ITAM)-containing domains such as DAP12 for the NCR and NKG2C, or CD3ζ and FcεRIγ for CD16a [43]. In the case of NKG2D, another receptor of the NKG2 family, it is associated with DAP10 [44]. In any case, when these activating receptors bind to their ligand, the intracellular chains will be phosphorylated by protein kinase from the Src family. This event will trigger various intracellular pathways including Ca2+ flux, ultimately leading to exocytosis and gene transcription [44]. On the other hand, inhibitory receptors signal through immunoreceptor tyrosine-based inhibitory motif (ITIM) domains. ITIM domains recruit phosphatases that dephosphorylate several targets, inhibiting NK cell function [44]. In this section, we will focus only on the activating receptors, responsible for NK cell antitumor functions.
Natural cytotoxic receptors
The members of the NCR family, namely NKp30, NKp44 and NKp46, are almost all ITAM-bearing receptors and thus activating receptors, except for some variants. In humans, six NKp30 variants have been described, some being activating and others potentially inhibitory [43]. Nonetheless, NKp30 was reported to induce NK cell activation and cytotoxicity upon binding to its ligands BAT3 [45] and B7-H6 [46]. Both antigens have been associated with poor outcomes in patients suffering from gastrointestinal cancer [47], and NK cells’ low NKp30 surface expression is linked to poor patients’ outcomes in hematological and solid cancer [48–51].
NKp44 can bind to PCNA, PDGF-DD and NID1 [43], 21spe-MLL5 and cell-surface heparan sulfate proteoglycans (HSPG) [52]. There are 3 different isoforms of NKp44: NKp44-1, -2 and -3. NKp44-1 possesses an ITIM domain in its cytoplasmic tail, while the two others have an ITAM domain. NKp44-1 binding to PCNA decreases NK cell activation and its expression is associated with low survival in acute myeloid leukemia patients [53]. In line with this, in vitro PCNA blockade using monoclonal antibodies (mAbs) enhances NK cells mediated tumor cell killing [54]. On the other hand, NKp44/PDGF-DD interaction induces cytokine production by NK cells. Moreover, NKp44 high expression is linked to increased survival in low grade glioma patients [55]. NKp44 binding to NID1, a component of the extracellular matrix, inhibits PDGF-DD-induced cytokines production [56]. Accordingly, NID1 expression is linked to poor patient outcomes in low-grade gliomas [57]. MLL5 is primarily a nuclear protein but its isoform, 21spe-MLL5, is found in the cytoplasm and at the cell surface of cancer cells. Its recognition by NKp44 trigger NK cell’s cytotoxicity [58]. Finally, NKp44 can recognize cell surface HSPG, whose expression vary between cell type and can be modified in tumor cells [59]. Although HSPG recognition by NKp44 triggers IFNγ secretion [60], other report tends to show that activation of a cytotoxic activity is most likely not happening [61].
NKp46 is involved in the killing of various tumor cells since NKp46 blockade using mAbs drastically decreased NK cell’s ability to destroy tumor cells [62]. Moreover, in several cancer types, low NKp46 transcription and surface expression on NK cells have been associated with poor patient outcomes [23, 63, 64]. In colorectal cancer patients, CD56dim NK cells express less NKp46 than in healthy donors, suggesting an anti-tumor role for NKp46 [65]. Nevertheless, the NKp46 ligand externalized calreticulin (ecto-CRT) was only recently described on tumor cell surface [66]. This protein is normally found in the endoplasmic reticulum (ER) where it acts as a chaperone. However, during ER stress induced by immunogenic cell death inducers, ecto-CRT is translocated to the plasma membrane [67]. Hence, chemotherapy-treated tumor cells express ecto-CRT at their membrane, becoming more sensitive to NK cell activity through, at least in part, ecto-CRT recognition by NKp46.
NKG2 family
Another main NK cell activating receptor is NKG2D, which binds to several ligands, some of them being referred to as “stress ligands” such as MHC class I chain-related proteins A and B (MICA/B) and the UL16 binding protein (ULBP) molecules family [68]. Intracellular signaling of NKG2D is mediated by DAP10, a non-bearing ITAM domain intracellular chain [44]. The importance of this receptor for tumor immunosurveillance was demonstrated using a murine model of NKG2D deficiency in which tumor incidence was highly increased [69]. In fact, NKG2D ligands have been found at the surface or tumor cells or in soluble form in a wide range of cancers, especially MICA [70]. More recently, NKG2D’s role in clearing circulating tumor cells was highlighted in a study showing that recognition of its cognate ligands is potentiated in fluid shear stress through mechanosensing [71]. In the clinic, a high level of NKG2D ligands’ expression at the tumor cells membrane is correlated with better prognosis and increased NK cell infiltration in the tumor [72]. Interestingly, a recent report demonstrated that CD8 + T cells are able to eliminate MHC-I negative tumor cells in mice model and in vitro with human cells through the NKG2D axis [73]. Thus, NKG2D-mediated tumor clearance could also be, at least in part, due to CD8 + T cells activity.
NKG2C is an activating receptor that works in heterodimers with CD94 [74]. CD94/NKG2C recognizes HLA-E which is expressed by several cell types including tumor cells [75]. CD94/NKG2C signaling is driven by an ITAM-bearing chain, DAP12 [74]. The amount of NKG2C-positive NK cells appears to be beneficial in hematopoietic cell transplant (HCT). First, they were associated with a better response after HCT in patients with hematological malignancies [76]. Next, NK cells are known to be involved in silencing graft-versus-host-disease (GvHD) mediated by the donor T cells by destroying them [77]. More specifically, the NKG2C-positive NK cells seem to be critical in this context, as patients with severe GvHD were found to have lower percentage of this NK cell subset [78]. Another potential ligand for CD94/NKG2C is membrane Heat shock protein 70 (Hsp70). This stress protein is expressed at an elevated level at the surface of several types of solid cancer cells [79] and was shown to sensitize them to NK cells activity [80]. In this context, CD94 was found to be involved in the recognition of Hsp70 [81].
DNAM1
DNAM1/CD226 is a potent activating receptor that has been shown to recognize two ligands overexpressed by tumor cells [82], namely PVR/CD155 which is considered as the main ligand [83] and Nectin2/CD112 [84]. CD155 expression correlates with bad prognosis in several solid cancers, pointing out the importance of this ligand for tumor control [85, 86]. Consistently, DNAM1 deficient mice show accelerated tumor growth compared to WT mice [87], although not only NK cells but also CD8+ T cells express this receptor [88]. Hence, the observed effect might be the sum of impede function of both NK cells and CD8+ T cells.
CD16a/FcγRIIIa
Last, but not least, the strongest degranulation inducer is probably the CD16a receptor, which belongs to the Fc-gamma receptor family, which includes receptors for the Fc moiety of IgG [89]. CD16a is a transmembrane receptor that possesses two extracellular immunoglobulin domains [90]. CD16a short cytoplasmic tail does not contain any signaling component; hence non-covalently interacting ITAM-bearing intracellular chains are needed to induce strong intracellular signals [91]. Hence, antibody-coated cells can be recognized by CD16a expressing NK cells, leading to NK cell degranulation. Of interest, a functional polymorphism has been described, the F158V, the V variant being the one with the best affinity for the Fc moiety of IgG [92].
Lytic granules: NK cells’ nuclear bomb
As described above, NK cells as well as other cytotoxic T lymphocytes carry lytic granules in their cytosol. These granules contain several cytotoxic molecules, such as Prf, GrzB and granulysin (GNLY). Interestingly, NK cells constitutively express these granules, whereas cytotoxic T lymphocytes require activation [93]. This notably could help to explain the rapid functions of NK cells in contrast to CD8+ T cells.
Recently, an article reviewed the steps leading to the biogenesis of GrzB [40]. Briefly, GrzB is produced as a zymogen composed of the catalytic part, a peptide signal and an inhibitory peptide. Once it arrives in the ER, it translocates to the Golgi apparatus and then into lytic granules. Inside lytic granules, the inhibitory dipeptide is cleaved, however, GrzB activity is still inhibited through interaction with a serglycin molecule. Concerning Prf, it is produced in the ER in an inactive state, and it translocates to the Golgi apparatus then in lytic granules [40]. The exact mechanism remains unclear thus further research is needed [40].
Upon release in the IS, Prf will form a pore into the target cell’s membrane allowing GrzB to penetrate the cytoplasm [94, 95]. GrzB will then initiate apoptosis by cleaving several substrates such as Bid or caspase-3 [96–98]. Of note, it is known that some target cells can be destroyed through necrosis, pointing out an osmotic lysis driven by Prf only [99]. Additionally, GNLY also displays pore-forming activities, allowing not only GrzB to enter target cells [100] but also directly trigger target cell’s apoptosis by inducing cytochrome C release from mitochondria [101].
Recently, it has been described that Prf and GrzB can be released in complex with thrombospondin-1 (TSP-1) from lytic granules, forming structures termed SMAPs standing for supramolecular attack particles. SMAPs may contribute to the efficiency of the delivery of GrzB and Prf to the target cell. Interestingly, SMAPs size from NK cells were found to be larger than those from T cells [102].
NK cells are protected from the Prf/GrzB cytotoxic activity after release through the CD107a/LAMP-1 translocation at the membrane. Originally present at the surface of the lytic granule, CD107a will be expressed at the surface plasma membrane after exocytosis and thus prevent pore-formation [103].
Finally, target cells can confine GrzB in the endosomal compartment as a defensive measure. However, Prf is capable of permeabilizing endosomes thus releasing the sequestered GrzB into the cytosol [104].
Delivering the kiss of death: TNF-related apoptosis-inducing ligands
Apart from lytic granules release, NK cells can rely on other weapons namely two TNF-related apoptosis-inducing ligands belonging to the TNF superfamily, TRAIL and FasL (CD95L). Upon binding to their cognate ligands, TRAIL R1–R2 and Fas receptor (CD95) respectively, apoptosis will occur inside the target cells [105, 106]. The apoptosis pathway triggered by death receptor engagement is the extrinsic pathway, contrary to cytotoxic molecules from lytic granules that trigger the intrinsic pathway. Fas and TRAIL ligands engagement induce a Death-Inducing Signaling Complex (DISC) formation mediating strong apoptotic intracellular signal through caspase 8 maturation and subsequently effector caspase 3, 6 and 7 activation [107].
FasL and TRAIL expression regulation are still a subject of research. FasL seems to be stored separately from Prf/GrzB in another type of lytic granule [108] and upon lytic granule-mediated target cell killing, is gradually expressed at the membrane [35, 109]. Regarding TRAIL, additional work is needed to decipher its intracellular localization and regulation in humans. Nevertheless, TRAIL surface expression seems to be IFNγ dependent, as demonstrated in IFNγ-deficient mouse models [110, 111]. Additionally, in a murine model, NKp46 has also been shown to regulate TRAIL membrane location. Indeed, NK cells from NKp46 deficient mice were devoid of membrane-bound TRAIL but were found intracellularly [112]. In humans NK cells, TRAIL surface expression is inducible through cytokines stimulation [113], similar to T cells on which TRAIL can only be found at the membrane after activation [114].
IFNγ and TNF-α: the right and left arms of NK cells
NK cells are also well-known cytokine producers, in particular IFNγ and TNF-α. Cytokines are stored in different vesicles than lytic granules and are released upon NK cell’s activation [115]. However, the secretion pathway is different since lytic granules are secreted locally in the IS as mentioned above, whereas cytokines are widely released in the environment [116].
As pro-inflammatory cytokines, IFNγ and TNF-α promote other immune cell activation and can induce tumor cell apoptosis [117, 118]. TNF-α increases IFNγ production through signaling with its receptors TNFR2 [119], while IFNγ increased the motility and cytotoxicity of cytotoxic lymphocytes [120]. Taken together, these studies highlight the essential role of cytokines in mediating and supporting NK cell cytotoxicity, as well as communicating with other immune cells.
Antibody-dependent-cell-cytotoxicity
Upon CD16a binding to IgG Fc, a potent cytotoxic mechanism known as Antibody-Dependent-Cell-Cytotoxicity (ADCC) is triggered, leading to target cell death. Although CD16a alone is enough to trigger ADCC, other receptors seem to positively modulate it as shown for LFA-1, CD38 or CD2 [121–123]. ADCC is one of the main mechanisms of action of some clinical mAbs. Indeed, patients harboring CD16 158 V NK cells show a better clinical response after rituximab (an antibody targeting CD20) treatment [124] and in trastuzumab (an antibody targeting HER2)-treated HER2+ breast cancer patients [125]. Moreover, it has been recently shown in mice models that high levels of intra-tumor hinge-cleaved IgG impaired NK cell function and tumor clearance, highlighting the importance of CD16a/IgG Fc interaction for NK cells anti-tumor activity [126]. In the clinic, tumor-infiltrating NK cells are predictive of pathological complete response in breast cancer patients who received anti-HER2 mAb [127]. In hematological cancer, peripheral blood NK cell count seems associated with follicular lymphoma and diffuse large B cell lymphoma (DLBCL) patients’ response to anti-CD20 mAb therapy [128]. Taken together, NK cell mediated ADCC appears to be an essential mechanism of several widely used clinical mAbs.
Dynamics of NK cell’s cytotoxic activity
NK cells rely on several killing pathways to achieve target cell elimination (Fig. 1). However, when, and how NK cells use which pathway is still an active field of study. After destroying a target cell, NK cells can find another target cell. This process is known as serial killing, in which NK cells destroy target cells one by one [129]. Primary NK cells carry about 60 lytic granules in their cytosol and they degranulate around 10% of their total granule per killing event [130]. This is consistent with previous findings showing that NK cells can kill up to 10 targets during serial killing [129]. Of note, 1% of NK cells’ lytic granules may be enough to kill a target cell, highlighting the exceptional potency of lytic granule content [130]. During serial killing, repeated stimulation of the CD16a pathway diminishes the amount of Prf secreted over time. Interestingly, switching the NK cells degranulation pathway to NKG2D restores the original amount of secreted Prf [131]. Thus, when NK cells use all available lytic granules, they switch their killing pathway toward death receptors-mediated cytotoxicity [113]. Indeed, FasL was shown to be expressed gradually at the membrane during serial killing concomitantly with CD107a expression, since it is stored in lytic granules. Interestingly, FasL-mediated target cell killing seems to account for only one, ultimate killing event [113]. Of note, death receptors-induced target cell apoptosis was shown to be a rather slow mechanism, while lytic granule-mediated cell death occurs faster [132].
Fig. 1. NK cell cytotoxic Arsenal.
NK cells rely on activating receptors which lead to lytic granules content and cytokines release and on death receptors whose induce apoptosis. GNLY granulysin, GrzB granzyme B, Prf perforin. Created with BioRender.com.
IS termination and subsequent NK cell detachment from a target cell seem to be a critical step in serial killing. It was proposed that NK cells can interact and integrate signals from several targets at the same time. Indeed, it was shown that the first killing event is slower than the next ones, and that consecutive killing events are adjacent [91]. In line with this, others described that upon interacting with a new target cell, NK cells will detach faster from the previous one [41]. Moreover, the detachment process was found to be dependent on the successful killing of the target cell, and a failure was associated with an extensive cytokines release, including IFNγ [133]. Interestingly, a similar pattern can be found in studies reporting ADAM17 blockade strategies. ADAM17 is a metalloprotease responsible for the CD16a shedding after ADCC induction [134, 135]. Hence, blocking ADAM17 activity could increase NK cells mediated ADCC. In the end, it was shown that blocking ADAM17 using chemicals, mAbs or by generating NK cells bearing non-cleavable CD16a not only increased NK cells IFNγ secretion during ADCC assays [135, 136] but also reduced serial killing [131]. These findings are consistent with the above-mentioned study and point out the critical role of IS disassembly in NK cells serial killing [133].
Analyzing NK cell interaction with tumor cells
The presence of NK cells that interact with tumor cells have recently been described in leukemia and lymphoma patients. NK cells interacting with tumor cells usually express the two CD45 isoforms CD45RA and CD45RO [137], and the interaction generates new NK cell subsets with different functionalities [138]. The anti-tumor NK cells perform trogocytosis on tumor markers facilitating their identification [137]. The following of this NK cell subset allows the surveying of the presence of tumor cells and the prognostic/theragnostic of the patient [139–141]. Moreover, trogocytosis can affect NK cell functionality [142–145] and, probably, the response to treatment [146].
NK cells crosstalk with other immune cells: need help from some friends
In the TME, the NK cell lineage act as a veritable conductor of the anti-tumor immune response. NK cells cooperate through direct contact or soluble factor secretion with other immune cells and participate in their recruitment, maturation, polarization, differentiation and destruction. In turn, immune cells can have a positive impact on NK cells by increasing ADCC, IFNγ production, activating receptor expression, recruitment and cytotoxicity. However, some cells could also have a negative impact on NK cells by reducing their activation and cytotoxicity.
NK cells cooperation with innate immune cells
Macrophages
Macrophages are phagocytic and antigen-presenting cells at the interface between innate and adaptive immunity. Tumor-associated macrophages (TAM) are divided into two subtypes: TAM1 with an anti-tumor activity and TAM2 with a pro-tumor activity. TAM1 are induced by IFNγ and LPS and produce cytokines such as TNF-α, IL-1β, IL-6, IL-12, nitric oxide synthase (iNOS) and reactive oxygen species (ROS). TAM2 are induced by cytokines such as IL-4, IL-10 and IL-13, and produce cytokines such as IL-10, TGF-β and VEGF [147, 148].
First, TAM1, by both soluble factor secretion and direct contact, increase CD69 expression (an activating marker), degranulation and IFNγ secretion of autologous resting NK cells [149]. The production of IFNγ by TAM induce the expression of IL-15Rα on NK cells and their IL-15 production. This cis-presentation increases the production of IFNγ by NK cells [149]. Interestingly, TAM1 also increase NK cell’s cytolytic efficiency by up-regulating NKG2D and NKp44 expression through IL-1β, IL-23 and IFN-β secretion [149]. Additionally, polyI:C-treated macrophages increase NKG2D expression on murine splenic CD49b+ NK cells and therefore, their cytotoxicity against several tumor cell lines. Interestingly, these murine macrophages express Qa-1 which is recognized by the inhibitory receptor NKG2A, thus avoiding to be killed by NK cells [150]. Monocyte-derived macrophages cultured with IFNλ/LPS produce IL-12 family cytokines which in turn induce human NK cells IFNγ production [151].
One study focused on the mechanism involved in NK cell-mediated tumor killing orchestrated by macrophages and dendritic cells (DCs). In particular, the C-type lectin receptor Dectin-1, which is expressed by macrophages and DCs, able recognition of N-glycan structures on tumor cells. This induces IRF5 activation and in turn downstream genes’ transcription. Overall, these events allow macrophages and DCs to activate NK cell’s anti-tumor activity against N-glycan-expressing tumor cells [152].
In contrast, TAM-derived TGF-β impairs NK cell function by decreasing their cytokine production, degranulation in humans [153, 154] and cytotoxicity in mice [155]. TAM also promote an exhausted phenotype CD27low CD11bhigh in mice [155]. In a mice model, Klose et al. demonstrated that targeting VEGF-A in myeloid cells increases chemotherapy delivery to the tumor bed and also increases NK cell recruitment after chemotherapy [156].
NK cells can also negatively affect anti-tumor macrophage function. For example, peripheral blood NK cells from prostate cancer patients exhibit an exhausted phenotype characterized by a decrease of NKG2D and an increase of PD-1 and TIM-3 expression [157]. Of note, PD-1 and TIM-3 are two inhibitory surface molecules. The most known inhibitory axis is PD-1 and its two ligands, PD-L1 and PD-L2, which are overexpressed in some cancers, inhibiting NK cells anti-tumor activity [158, 159]. Consistently, NK cells from prostate cancer patients show a greatly diminished degranulation capacity. These NK cells also secrete soluble factors involved in monocyte recruitment and M2-polarization such as CCL2 and IL-10 [157] (Fig. 2).
Fig. 2. NK cells and macrophage interaction.
Macrophages are innate immune cells divided into pro-tumor M1 macrophage and anti-tumor M2 macrophages. There are different interactions between NK cells and macrophages. Macrophages and notably M1 have an anti-tumor effect on NK cells: increase of activating receptors, cytokine production and degranulation. Macrophages also have a pro-tumor effect by inhibiting NK recruitment, degranulation, cytokine production and inducing an exhaustion phenotype. In turn, NK cells induce monocyte recruitment and M2 polarization. Created with BioRender.com.
Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSC) are divided into two groups: polymorphonuclear MDSC (PMN-MDSC), derived from the granulocytic lineage, and monocytic MDSC (M-MDSC) that originate from the myeloid lineage. These cells are known to induce an immunosuppressive TME: M-MDSC through Arginase-1, Nitric Oxide, IL-10 and TGF-β and PMN-MDSC through reactive oxygen species (ROS) and reactive nitrogen species (RNS) production [160].
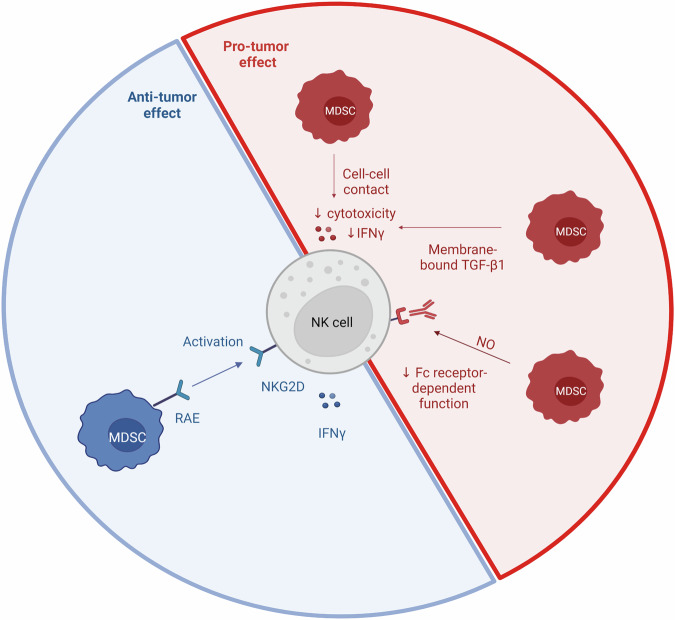
MDSCs can reduce NK cell’s cytotoxicity. MDSCs from patients with hepatocellular carcinoma inhibit autologous NK cell cytotoxicity and IFNγ production through an NKp30 interaction [161] or by their membrane-bound TGF-β1 [162]. MDSCs also inhibit the Fc receptor-dependent function of NK cells by producing NO [163].
However, Nausch et al. demonstrated that MDSC can also activate NK cells through their expression of RAE, a ligand of the activating receptor NKG2D. In fact, they showed that NK cells from naive mice co-culture with MDSC produce more IFNγ. This increase was partially reduced using transwell and NKG2D blocking antibodies. Thus, IFNγ production by NK cells was dependent on NKG2D signaling [164] (Fig. 3).
Fig. 3. NK cells and MDSC interaction.
MDSC are innate immune cells divided into PMN-MDSC and M-MDSC. There are different interactions between MDSC and NK. MDSCs reduce the cytotoxicity, the IFNγ production of NK and their Fc-receptor dependent function. MDSCs also have an anti-tumor effect by expressing Rae, which binds to NKG2D and induces the production of IFNγ by NK cells. Created with BioRender.com.
Dendritic cells
DCs are innate immune cells that link innate and adaptive immunity through their role as antigen-presenting cells. Through their dendrites, DCs recognize damage-associated molecular patterns (DAMPs), activate and mature. Next, they migrate to T-lymphocyte-rich secondary lymphoid organs, where they present an antigen peptide through the major histocompatibility complex class II (MHC-II). Lymphocytes specific to the antigen peptide will activate, proliferate, and move towards the tumor site. In 1999, an initial study suggested that DCs were capable of initiating NK cell-mediated anti-tumor responses [165]. Subsequent studies showed that DCs and NK cells interact and the last can promote the maturation of DCs, the death of the immature ones and their recruitment to the tumor site. In addition, DCs promote NK cells anti-tumor activity.
NK cells modulate the number of DCs in tumors by producing CCL5 and XCL1, which recruit conventional type 1 dendritic cell (cDC1) [166]. Another study showed an increase in CD103+ DCs infiltration in CCL3-secreting CT26 tumor in comparison to WT CT26. CCL3 recruits NK cells, which in turn induced DCs recruitment. Indeed, DCs accumulation was abolished using an anti-Asialo-GM1 antibody, suggesting a role of NK cells in CD103+ DCs recruitment [167]. Barry et al. demonstrated that NK cells produce Flt3L, which increase intratumor stimulatory DCs number in mice tumors. Furthermore, the authors found that high levels of NK cells and BDCA3+/CD141+ DCs (which are intratumoral type I conventional dendritic cells) positively correlate with overall survival and PD-1 blockade outcome in patients suffering from melanoma. Finally, BDCA3+ DC/NK cell interaction was also found in humans head and neck squamous cell carcinoma, suggesting that this mechanism could exist as well in other cancer types [168].
Furthermore, NK cells contribute to DC maturation in several way. Firstly, self-specific MHC-I expressing NK cells aid DC maturation in a TNFSF14/LIGHT-dependent manner [169]. Moreover, TNF-α and IFNγ produced in an NKp30-dependent manner by NK cells also contribute to DC maturation [170]. Moreover, coculture with NK cells purified from lung cancer patients containing a subset of CTLA-4+ NK cells and DCs from the blood of the same patients results in a decrease of MHC-II and CD86 expression on DCs. This decrease was partially rescued when an anti-CTLA-4 antibody was added to the coculture, suggesting an inhibitory role of CTLA-4-expressing intratumor NK cells [171].
Finally, DCs can promote antibody-mediated NK cell activation [172] and Jagged2 and Notch could be responsible [173]. Secretion of IL-10 by BDCA1+ myeloid DCs and IL-6 by BDCA4+ plasmacytoid DCs could in part, be responsible for the inhibition of NK cells cytokine production and cytotoxicity [174] (Fig. 4).
Fig. 4. NK cells and DC interaction.
DCs are able to increase antibody-mediated NK cell activation but in some other conditions to decrease NK cytokines production and cytotoxicity. In the other part, NK recruits DC and increases their maturation. However, the expression of some immune checkpoints on NK cells is able to decrease DC maturation. Created with BioRender.com.
Neutrophils
Neutrophils are the most abundant immune cells in the blood: they account for 50-70% of circulating leukocytes in humans. In tumors, they are classified into two phenotypes: an N1 neutrophil phenotype with anti-tumor activity, which induces direct or indirect cytotoxicity by producing TNF-α, CCL3 and expressing ICAM-1. N2 neutrophils are anti-tumor, promote immunosuppression, angiogenesis and produce various cytokines: VEGF, MMP9 CXCL1,2,8,16 and CCL2,3,4,8,12 and 17 [175].
Using the MCA205-Luc mice model, researchers demonstrated that NK cells control tumor growth. Interestingly, in the absence of NK cells, neutrophils over-express VEGF-A and promote angiogenesis. In this context, NK cells seem to limit the pro-tumor effects of neutrophils [176].
Neutrophils can increase NK cell activation. In humans, neutrophils increase IFNγ production by NK cells indirectly by increasing IL-12p70 release by 6-sulfo LacNAc+ DCs (slanDCs), a major DCs subset found in the blood and tissues [177] and directly via CD18 and ICAM-3 interaction [178]. Neutrophils also show a negative effect on NK cells as neutrophils can inhibit NK cell functions through several mechanisms. First, PD-L1 expressing neutrophils can reduce PD-1+ NK cells cytotoxicity and infiltration into tumors [179]. Moreover, neutrophils also produce cathepsin G which cleaves NKp46 on the NK cell’s surface, diminishing their cytotoxic function [180] (Fig. 5).
Fig. 5. NK cells and neutrophils interaction.
Neutrophils are able to increase NK cell IFNγ production directly or indirectly through slanDCs IL-12p70 production. On the other hand, neutrophils can decrease NK cells cytotoxicity and recruitment in tumor. Created with BioRender.com.
NK cells cooperation with adaptative immune cells
Mucosal-associated invariant T cells
Mucosal-associated invariant T cells (MAITs) are unconventional T lymphocytes possessing an invariant TCR that exclusively recognizes the MR1 protein presented by MCH class I (MHC-I) [181]. Activation of MAITs occurs either dependently or independently of TCR recognition and results in cytokines and cytotoxic effectors secretion.
A recent study showed that MAITs play a role in regulating the anti-tumor effect mediated by NK cells. In fact, it initially demonstrated that the absence of MAIT strengthens the antitumor effect of NK cells. However, this role seems controversial because it also showed that activated MAIT cells increased NK cell’s anti-tumor functions through IFNγ secretion [182].
CD4+ T cells
CD4+ T-lymphocytes (CD4+ T cells) are adaptive immune cells that recognize antigenic peptides associated with MHC-II. Classically, MHC-II expressing cells are antigen-presenting cells: DCs, macrophages and B cells. When T cells are activated, they proliferate and, depending on the cytokine environment, differentiate into a subpopulation: Th1, Th2, Th17, regulatory T cells (Treg), follicular helper T cells (Tfh). These subpopulations will orchestrate the immune response by producing cytokines [183].
Mouse NK cells modulate MHC-II-dependent CD4+ T cell responses because NK cells can acquire MHC-II by trogocytosis during direct contact with DCs in vivo. NK cells co-cultured with DCs also expressed low levels of CD80 and CD86, but not enough to be functional [184]. Hence, these NK cells suppress DCs-induced CD4+ T cells responses by competing with antigen presentation [184]. The interaction between DCs and NK cells also replaces the help of CD4+ T cells in the priming of cytotoxic T lymphocytes [185]. NK cells may also interfere with Tregs, which are immunosuppressive T cells expressing CD4+ FOXP3+ CD25+. In fact, differentiation of Tregs can be prevented by activated NK cells, which interfere with CD28 signaling in CD4+ CD25- T cells and inhibit Foxp3 transcription [186]. NK cell depletion also impairs Th1 polarization [187].
In another study, NK cells and CD4+ T cells were found to work together to protect mice from melanoma tumor formation in the brain [188]. T cells and notably CD4+ T cells help maintain NK cell viability and improve rituximab-mediated NK cells ADCC [189].
In 1999 [190], 2003 [191] and 2004 [192] three different studies showed that Tregs could inhibit NK cell’s functions. The mechanism of interaction was deciphered in 2005 and showed that Tregs inhibit NK cell’s lytic and secretory functions in a TGF-β-dependent manner [193] (Fig. 6).
Fig. 6. NK cells and T cells interaction.
NK are able to suppress DCs-induced CD4+ T cells response, to block Treg differentiation, to promote CD8+ T cells recruitment directly or indirectly via iDC interaction and to restrict TEM CD8+ T cells generation. On their side, adaptive immune cells are able to maintain NK viability, to drive NK maturation, and improve NK cells ADCC. Created with BioRender.com.
CD8+ T cells
NK cells can impact the CD8+ T cells response by interacting with DCs, as described above. Moreover, in certain conditions, IL-18-primed NK cells attract iDCs by producing chemokines such as CCL3 and CCL4. In co-culture with iDC, NK cells induce the production of T cells recruiting chemokines such as CXCL9, CXCL10 and CCL5 by iDC [194]. However, NK cells can also restrict TEM CD8+ T cell generation indirectly through PD-1/PD-L1 interaction with DC [195].
CD8 + T cells can also drive NK cells maturation. Indeed, in a mice model devoid of CD8 + T cells, liver-resident NK cells appear to be immature, and this can be reverted by CD8 + T cells replenishment. Liver-resident NK cell maturation by CD8 + T cells depends on the CD27/CD70 axis [196].
NK cells can shape the anti-tumor immune response by recruiting T cells through cytokines production. As early as 1994, it was demonstrated that NK cells play a role in T cell recruitment [197]. Indeed, in response to antibody-coated tumor cells, NK cells produce IL-8, MDC, MIP-1 and CCL2, which are chemokines involved in T cells recruitment [198]. Recently, NK cells were found to induce CD8 + T cells recruitment to metastatic site in the B16F10 lung metastasis melanoma model, although the underlying mechanism was not deciphered [28]. Finally, T cells have been also identified to help the anti-tumor immune response mediated by NK cells [199] (Fig. 6).
Conclusion
Since the first report of “naturally occurring killer lymphocytes”, NK cells have been extensively studied by the scientific community. Their innate ability of recognizing abnormal cells, and their broad cytotoxic arsenal make them an important protagonist in the fight against tumor. Studying how, when and why NK cells target and destroy tumor cells is a key in designing innovative drug to improve NK cells activity. In the tumor bed, NK cells interact with other immune cells through cell-cell contact or cytokines secretion, inducing beneficial or harmful response. Deciphering these interactions could bring new treatment options by targeting new activating or inhibiting pathways to maintain or refuel NK cells activity.
NK cells are gaining more and more attention with the rise of NK-cell-based therapy, CAR-modified or not. Indeed, their safety profile in clinic and the possibility to use them in heterologous settings are two major advantages compared to CAR-T cell therapy. However, their clinical efficacy in monotherapy is deceiving and there is a need to develop new cotreatments [200]. The knowledge summarized here could help to improve NK cells’ cytotoxic activity as well as predict how ex vivo expanded NK cells infused in patients will behave in the tumor bed.
Acknowledgements
We acknowledge the imaging facility MRI, a member of the National Infrastructure France-BioImaging supported by the French National Research Agency (ANR-10-INBS-04, Investments for the future).
Author contributions
LC and MG wrote the manuscript. FG, MV and MB revised and wrote the manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript. The order of the firsts co-authors was determined by alphabetical order.
Funding
This work was supported by “Institut National Du Cancer” (INCA) PRT-K program 2021 (MV, 2021-014). The “Investissements d’avenir” Grant LabEx MAbImprove: ANR-10-LABX-53 (MV). The 2021 AAP Companies On Campus by the Montpellier Université d’Excellence (MUSE; MV). LC is a recipient of a fellowship from MRT and from “Fondation pour la Recherche Médicale” (FDT202304016765). MG is recipient of a fellowship from MRT and from “Fondation pour la Recherche Médicale” (FDT202304016666). MB is supported by the ARC foundation (ARCPJA2021060003768) and the Ligue contre le cancer.
Data availability
The relevant information is available from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Edited by: Francesca Pentimalli
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Loïs Coënon, Mannon Geindreau.
References
- 1.Kiessling R, Klein E, Wigzell H. ‘’Natural’’ killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–7. 10.1002/eji.1830050208 [DOI] [PubMed] [Google Scholar]
- 2.Kiessling R, Petranyi G, Klein G, Wigzel H. Genetic variation of in vitro cytolytic activity and in vivo rejection potential of non-immunized semi-syngeneic mice against a mouse lymphoma line. Int J Cancer. 1975;15:933–40. 10.1002/ijc.2910150608 [DOI] [PubMed] [Google Scholar]
- 3.Savoy SKA, Boudreau JE. The evolutionary arms race between virus and NK cells: diversity enables population-level virus control. Viruses. 2019;11:959. 10.3390/v11100959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19:120. 10.1186/s12943-020-01238-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu M, Liang S, Zhang C. NK cells in autoimmune diseases: protective or pathogenic? Front Immunol. 2021;12:624687. 10.3389/fimmu.2021.624687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kucuksezer UC, Aktas Cetin E, Esen F, Tahrali I, Akdeniz N, Gelmez MY, et al. The role of natural killer cells in autoimmune diseases. Front Immunol. 2021;12:622306. 10.3389/fimmu.2021.622306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Wang F, Imani S, Tao L, Deng Y, Cai Y. Natural killer cells: friend or foe in metabolic diseases? Front Immunol. 2021;12:614429. 10.3389/fimmu.2021.614429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romagnani C, Juelke K, Falco M, Morandi B, D’Agostino A, Costa R, et al. CD56brightCD16− killer Ig-like receptor− NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–55. 10.4049/jimmunol.178.8.4947 [DOI] [PubMed] [Google Scholar]
- 9.Chan A, Hong DL, Atzberger A, Kollnberger S, Filer AD, Buckley CD, et al. CD56bright human NK cells differentiate into cd56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. 10.4049/jimmunol.179.1.89 [DOI] [PubMed] [Google Scholar]
- 10.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–51. 10.1182/blood.V97.10.3146 [DOI] [PubMed] [Google Scholar]
- 11.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol Balt Md 1950. 1989;143:3183–91. [PubMed] [Google Scholar]
- 12.Björkström NK, Ljunggren HG, Michaëlsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol. 2016;16:310–20. 10.1038/nri.2016.34 [DOI] [PubMed] [Google Scholar]
- 13.Wagner JA, Rosario M, Romee R, Berrien-Elliott MM, Schneider SE, Leong JW, et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest. 2017;127:4042–58. 10.1172/JCI90387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol. 2013;34:573–82. 10.1016/j.it.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crinier A, Milpied P, Escalière B, Piperoglou C, Galluso J, Balsamo A, et al. High-dimensional single-cell analysis identifies organ-specific signatures and conserved NK cell subsets in humans and mice. Immunity. 2018;49:971–986.e5. 10.1016/j.immuni.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. 10.1016/j.immuni.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong P, Foltz JA, Chang L, Neal CC, Yao T, Cubitt CC, et al. T-BET and EOMES sustain mature human NK cell identity and antitumor function. J Clin Invest. 2023;133:e162530. 10.1172/JCI162530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persyn E, Wahlen S, Kiekens L, Van Loocke W, Siwe H, Van Ammel E, et al. IRF2 is required for development and functional maturation of human NK cells. Front Immunol. 2022;13:1038821. 10.3389/fimmu.2022.1038821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crinier A, Narni-Mancinelli E, Ugolini S, Vivier E. SnapShot: natural killer cells. Cell. 2020;180:1280–1280.e1. 10.1016/j.cell.2020.02.029 [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci. 2000;97:2731–6. 10.1073/pnas.050588297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet Lond Engl. 2000;356:1795–9. 10.1016/S0140-6736(00)03231-1 [DOI] [PubMed] [Google Scholar]
- 22.Seymour F, Cavenagh JD, Mathews J, Gribben JG. NK cells CD56bright and CD56dim subset cytokine loss and exhaustion is associated with impaired survival in myeloma. Blood Adv. 2022;6:5152–9. 10.1182/bloodadvances.2022007905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafer D, Smith MR, Borghaei H, Millenson MM, Li T, Litwin S, et al. Low NK cell counts in peripheral blood are associated with inferior overall survival in patients with follicular lymphoma. Leuk Res. 2013;37:1213–5. 10.1016/j.leukres.2013.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nersesian S, Schwartz SL, Grantham SR, MacLean LK, Lee SN, Pugh-Toole M, et al. NK cell infiltration is associated with improved overall survival in solid cancers: A systematic review and meta-analysis. Transl Oncol. 2021;14:100930. 10.1016/j.tranon.2020.100930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichise H, Tsukamoto S, Hirashima T, Konishi Y, Oki C, Tsukiji S, et al. Functional visualization of NK cell-mediated killing of metastatic single tumor cells. Rothlin CV, editor. eLife. 2022;11:e76269. 10.7554/eLife.76269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020;30:764–76. 10.1016/j.tcb.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chockley PJ, Chen J, Chen G, Beer DG, Standiford TJ, Keshamouni VG. Epithelial-mesenchymal transition leads to NK cell–mediated metastasis-specific immunosurveillance in lung cancer. J Clin Invest. 2018;128:1384–96. 10.1172/JCI97611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyas M, Requesens M, Nguyen TH, Peigney D, Azin M, Demehri S. Natural killer cells suppress cancer metastasis by eliminating circulating cancer cells. Front Immunol. 2023;13:1098445. 10.3389/fimmu.2022.1098445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Zhao Z, Li F. Natural killer cell dysfunction in cancer and new strategies to utilize NK cell potential for cancer immunotherapy. Mol Immunol. 2022;144:58–70. 10.1016/j.molimm.2022.02.015 [DOI] [PubMed] [Google Scholar]
- 30.Dean I, Lee CYC, Tuong ZK, Li Z, Tibbitt CA, Willis C, et al. Rapid functional impairment of natural killer cells following tumor entry limits anti-tumor immunity. Nat Commun. 2024;15:683. 10.1038/s41467-024-44789-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mace EM, Dongre P, Hsu HT, Sinha P, James AM, Mann SS, et al. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol Cell Biol. 2014;92:245–55. 10.1038/icb.2013.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mace EM, Zhang J, Siminovitch KA, Takei F. Elucidation of the integrin LFA-1–mediated signaling pathway of actin polarization in natural killer cells. Blood. 2010;116:1272–9. 10.1182/blood-2009-12-261487 [DOI] [PubMed] [Google Scholar]
- 33.Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol Balt Md 1950. 2004;173:3653–9. [DOI] [PubMed] [Google Scholar]
- 34.Brown ACN, Dobbie IM, Alakoskela JM, Davis I, Davis DM. Super-resolution imaging of remodeled synaptic actin reveals different synergies between NK cell receptors and integrins. Blood. 2012;120:3729–40. 10.1182/blood-2012-05-429977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–12. 10.1084/jem.20051143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nitta T, Yagita H, Sato K, Okumura K. Involvement of CD56 (NKH-1/Leu-19 antigen) as an adhesion molecule in natural killer-target cell interaction. J Exp Med. 1989;170:1757–61. 10.1084/jem.170.5.1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunesch JT, Dixon AL, Ebrahim TA, Berrien-Elliott MM, Tatineni S, Kumar T, et al. CD56 regulates human NK cell cytotoxicity through Pyk2. eLife. 2020;9:e57346. 10.7554/eLife.57346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang MS, Hu Y, Sanchez EE, Xie X, Roy NH, de Jesus M, et al. Mechanically active integrins target lytic secretion at the immune synapse to facilitate cellular cytotoxicity. Nat Commun. 2022;13:3222. 10.1038/s41467-022-30809-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–25. 10.1038/nri2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ham H, Medlyn M, Billadeau DD. Locked and loaded: mechanisms regulating natural killer cell lytic granule biogenesis and release. Front Immunol. 2022;13:871106. 10.3389/fimmu.2022.871106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Netter P, Anft M, Watzl C. Termination of the activating NK cell immunological synapse is an active and regulated process. J Immunol Balt Md 1950. 2017;199:2528–35. [DOI] [PubMed] [Google Scholar]
- 42.Chan CJ, Smyth MJ, Martinet L. Molecular mechanisms of natural killer cell activation in response to cellular stress. Cell Death Differ. 2014;21:5–14. 10.1038/cdd.2013.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medjouel Khlifi H, Guia S, Vivier E, Narni-Mancinelli E. Role of the ITAM-bearing receptors expressed by natural killer cells in cancer. Front Immunol. 2022;13:898745. 10.3389/fimmu.2022.898745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. 10.1038/ni1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–74. 10.1016/j.immuni.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 46.Cao G, Wang J, Zheng X, Wei H, Tian Z, Sun R. Tumor therapeutics work as stress inducers to enhance tumor sensitivity to natural killer (NK) cell cytolysis by up-regulating NKp30 ligand B7-H6. J Biol Chem. 2015;290:29964–73. 10.1074/jbc.M115.674010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rusakiewicz S, Perier A, Semeraro M, Pitt JM, Pogge von Strandmann E, Reiners KS, et al. NKp30 isoforms and NKp30 ligands are predictive biomarkers of response to imatinib mesylate in metastatic GIST patients. OncoImmunology. 2017;6:e1137418. 10.1080/2162402X.2015.1137418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chretien AS, Fauriat C, Orlanducci F, Rey J, Borg GB, Gautherot E, et al. NKp30 expression is a prognostic immune biomarker for stratification of patients with intermediate-risk acute myeloid leukemia. Oncotarget. 2017;8:49548–63. 10.18632/oncotarget.17747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–22. 10.1172/JCI45816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costello RT, Knoblauch B, Sanchez C, Mercier D, Le Treut T, Sébahoun G. Expression of natural killer cell activating receptors in patients with chronic lymphocytic leukaemia. Immunology. 2012;135:151–7. 10.1111/j.1365-2567.2011.03521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutierrez‐Silerio GY, Bueno‐Topete MR, Vega‐Magaña AN, Bastidas‐Ramirez BE, Gutierrez‐Franco J, Escarra‐Senmarti M, et al. Non‐fitness status of peripheral NK cells defined by decreased NKp30 and perforin, and increased soluble B7H6, in cervical cancer patients. Immunology. 2023;168:538–53. 10.1111/imm.13593 [DOI] [PubMed] [Google Scholar]
- 52.Parodi M, Favoreel H, Candiano G, Gaggero S, Sivori S, Mingari MC, et al. NKp44-NKp44 ligand interactions in the regulation of natural killer cells and other innate lymphoid cells in humans. Front Immunol. 2019;10:719. 10.3389/fimmu.2019.00719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shemesh A, Brusilovsky M, Hadad U, Teltsh O, Edri A, Rubin E, et al. Survival in acute myeloid leukemia is associated with NKp44 splice variants. Oncotarget. 2016;7:32933–45. 10.18632/oncotarget.8782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marrufo AM, Mathew SO, Chaudhary P, Malaer JD, Ahmed N, Vishwanatha JK, et al. Blocking PCNA interaction with NKp44 enhances primary natural killer cell-mediated lysis of triple-negative breast cancer cells. Am J Cancer Res. 2023;13:1082–90. [PMC free article] [PubMed] [Google Scholar]
- 55.Barrow AD, Edeling MA, Trifonov V, Luo J, Goyal P, Bohl B, et al. Natural killer cells control tumor growth by sensing a growth factor. Cell. 2018;172:534–548.e19. 10.1016/j.cell.2017.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaggero S, Bruschi M, Petretto A, Parodi M, Del Zotto G, et al. Nidogen-1 is a novel extracellular ligand for the NKp44 activating receptor. Oncoimmunology. 2018;7:e1470730. 10.1080/2162402X.2018.1470730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang B, Xu C, Liu J, Yang J, Gao Q, Ye F. Nidogen-1 expression is associated with overall survival and temozolomide sensitivity in low-grade glioma patients. Aging. 2021;13:9085–107. 10.18632/aging.202789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baychelier F, Sennepin A, Ermonval M, Dorgham K, Debré P, Vieillard V. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood. 2013;122:2935–42. 10.1182/blood-2013-03-489054 [DOI] [PubMed] [Google Scholar]
- 59.Blackhall FH, Merry CLR, Davies EJ, Jayson GC. Heparan sulfate proteoglycans and cancer. Br J Cancer. 2001;85:1094–8. 10.1054/bjoc.2001.2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hershkovitz O, Jivov S, Bloushtain N, Zilka A, Landau G, Bar-Ilan A, et al. Characterization of the recognition of tumor cells by the natural cytotoxicity receptor, NKp44. Biochemistry. 2007;46:7426–36. 10.1021/bi7000455 [DOI] [PubMed] [Google Scholar]
- 61.Rossi GR, Gonçalves JP, McCulloch T, Delconte RB, Hennessy RJ, Huntington ND, et al. The antitumor effect of heparin is not mediated by direct NK cell activation. J Clin Med. 2020;9:2666. 10.3390/jcm9082666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29:1656–66. [DOI] [PubMed] [Google Scholar]
- 63.Chretien AS, Devillier R, Fauriat C, Orlanducci F, Harbi S, Le Roy A, et al. NKp46 expression on NK cells as a prognostic and predictive biomarker for response to allo-SCT in patients with AML. Oncoimmunology. 2017;6:e1307491. 10.1080/2162402X.2017.1307491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia-Iglesias T, Del Toro-Arreola A, Albarran-Somoza B, Del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Dueñas MG, et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186. 10.1186/1471-2407-9-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunol Immunother. 2019;68:1011–24. 10.1007/s00262-019-02343-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sen Santara S, Lee DJ, Crespo Â, Hu JJ, Walker C, Ma X, et al. The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells. Nature. 2023;616:348–56. 10.1038/s41586-023-05912-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu P, Zhao L, Kepp O, Kroemer G. Quantitation of calreticulin exposure associated with immunogenic cell death. Methods Enzymol. 2020;632:1–13. 10.1016/bs.mie.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 68.Textor S, Fiegler N, Arnold A, Porgador A, Hofmann TG, Cerwenka A. Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res. 2011;71:5998–6009. 10.1158/0008-5472.CAN-10-3211 [DOI] [PubMed] [Google Scholar]
- 69.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. 10.1016/j.immuni.2008.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhar P, Wu JD. NKG2D and its ligands in cancer. Curr Opin Immunol. 2018;51:55–61. 10.1016/j.coi.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu B, Xin Y, Hu G, Li K, Tan Y. Fluid shear stress enhances natural killer cell’s cytotoxicity toward circulating tumor cells through NKG2D-mediated mechanosensing. APL Bioeng. 2023;7:036108. 10.1063/5.0156628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGilvray RW, Eagle RA, Watson NFS, Al-Attar A, Ball G, Jafferji I, et al. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin Cancer Res J Am Assoc Cancer Res. 2009;15:6993–7002. 10.1158/1078-0432.CCR-09-0991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lerner EC, Woroniecka KI, D’Anniballe VM, Wilkinson DS, Mohan AA, Lorrey SJ, et al. CD8+ T cells maintain killing of MHC-I-negative tumor cells through the NKG2D–NKG2DL axis. Nat Cancer. 2023;4:1258–72. 10.1038/s43018-023-00600-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. 10.1016/S1074-7613(00)80574-9 [DOI] [PubMed] [Google Scholar]
- 75.Coupel S, Moreau A, Hamidou M, Horejsi V, Soulillou JP, Charreau B. Expression and release of soluble HLA-E is an immunoregulatory feature of endothelial cell activation. Blood. 2007;109:2806–14. 10.1182/blood-2006-06-030213 [DOI] [PubMed] [Google Scholar]
- 76.Cichocki F, Cooley S, Davis Z, DeFor TE, Schlums H, Zhang B, et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 2016;30:456–63. 10.1038/leu.2015.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–301. 10.1182/blood-2009-05-222190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kordelas L, Steckel NK, Horn P, Beelen D, Rebmann V. The activating NKG2C receptor is significantly reduced in NK cells after allogeneic stem cell transplantation in patients with severe graft-versus-host disease. Int J Mol Sci. 2016;17:1797. 10.3390/ijms17111797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, et al. A stress‐inducible 72‐kDa heat‐shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–9. 10.1002/ijc.2910610222 [DOI] [PubMed] [Google Scholar]
- 80.Botzler C, Issels R, Multhoff G. Heat-shock protein 72 cell-surface expression on human lung carcinoma cells is associated with an increased sensitivity to lysis mediated by adherent natural killer cells. Cancer Immunol Immunother. 1996;43:226–30. 10.1007/s002620050326 [DOI] [PubMed] [Google Scholar]
- 81.Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–79. 10.1515/BC.2003.030 [DOI] [PubMed] [Google Scholar]
- 82.Paolini R, Molfetta R. Dysregulation of DNAM-1-mediated NK cell anti-cancer responses in the tumor microenvironment. Cancers. 2023;15:4616. 10.3390/cancers15184616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tahara-Hanaoka S. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112). Int Immunol. 2004;16:533–8. 10.1093/intimm/dxh059 [DOI] [PubMed] [Google Scholar]
- 84.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as Cell Surface Ligands for the Human DNAM-1 (CD226) Activating Molecule. J Exp Med. 2003;198:557–67. 10.1084/jem.20030788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guillamón CF, Martínez-Sánchez MV, Gimeno L, Mrowiec A, Martínez-García J, Server-Pastor G, et al. NK cell education in tumor immune surveillance: DNAM-1/KIR receptor ratios as predictive biomarkers for solid tumor outcome. Cancer Immunol Res. 2018;6:1537–47. 10.1158/2326-6066.CIR-18-0022 [DOI] [PubMed] [Google Scholar]
- 86.Guillamón CF, Martínez-Sánchez MV, Gimeno L, Campillo JA, Server-Pastor G, Martínez-García J, et al. Activating KIRs on Educated NK Cells Support Downregulation of CD226 and Inefficient Tumor Immunosurveillance. Cancer Immunol Res. 2019;7:1307–17. 10.1158/2326-6066.CIR-18-0847 [DOI] [PubMed] [Google Scholar]
- 87.Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, Honda Sichiro, et al. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med. 2008;205:2959–64. 10.1084/jem.20081611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med. 2008;205:2965–73. 10.1084/jem.20081752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- 90.Woof JM, Burton DR. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol. 2004;4:89–99. 10.1038/nri1266 [DOI] [PubMed] [Google Scholar]
- 91.Blázquez-Moreno A, Park S, Im W, Call MJ, Call ME, Reyburn HT. Transmembrane features governing Fc receptor CD16A assembly with CD16A signaling adaptor molecules. Proc Natl Acad Sci USA. 2017;114:E5645–54. 10.1073/pnas.1706483114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, et al. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–70. 10.1172/JCI119616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nowacki TM, Kuerten S, Zhang W, Shive CL, Kreher CR, Boehm BO, et al. Granzyme B production distinguishes recently activated CD8(+) memory cells from resting memory cells. Cell Immunol. 2007;247:36–48. 10.1016/j.cellimm.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Law RHP, Lukoyanova N, Voskoboinik I, Caradoc-Davies TT, Baran K, Dunstone MA, et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature. 2010;468:447–51. 10.1038/nature09518 [DOI] [PubMed] [Google Scholar]
- 95.Lopez JA, Susanto O, Jenkins MR, Lukoyanova N, Sutton VR, Law RHP, et al. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood. 2013;121:2659–68. 10.1182/blood-2012-07-446146 [DOI] [PubMed] [Google Scholar]
- 96.Andrade F, Roy S, Nicholson D, Thornberry N, Rosen A, Casciola-Rosen L. Granzyme B directly and efficiently cleaves several downstream caspase substrates: implications for CTL-induced apoptosis. Immunity. 1998;8:451–60. 10.1016/S1074-7613(00)80550-6 [DOI] [PubMed] [Google Scholar]
- 97.Sutton VR, Davis JE, Cancilla M, Johnstone RW, Ruefli AA, Sedelies K, et al. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J Exp Med. 2000;192:1403–14. 10.1084/jem.192.10.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barry M, Heibein JA, Pinkoski MJ, Lee SF, Moyer RW, Green DR, et al. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol Cell Biol. 2000;20:3781–94. 10.1128/MCB.20.11.3781-3794.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zychlinsky A, Zheng LM, Liu CC, Young JD. Cytolytic lymphocytes induce both apoptosis and necrosis in target cells. J Immunol Balt Md 1950. 1991;146:393–400. [PubMed] [Google Scholar]
- 100.Anderson DH, Sawaya MR, Cascio D, Ernst W, Modlin R, Krensky A, et al. Granulysin crystal structure and a structure-derived lytic mechanism. J Mol Biol. 2003;325:355–65. 10.1016/S0022-2836(02)01234-2 [DOI] [PubMed] [Google Scholar]
- 101.Kaspar AA, Okada S, Kumar J, Poulain FR, Drouvalakis KA, Kelekar A, et al. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J Immunol. 2001;167:350–6. 10.4049/jimmunol.167.1.350 [DOI] [PubMed] [Google Scholar]
- 102.Ambrose AR, Hazime KS, Worboys JD, Niembro-Vivanco O, Davis DM. Synaptic secretion from human natural killer cells is diverse and includes supramolecular attack particles. Proc Natl Acad Sci. 2020;117:23717–20. 10.1073/pnas.2010274117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohnen A, Chiang SC, Stojanovic A, Schmidt H, Claus M, Saftig P, et al. Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood. 2013;122:1411–8. 10.1182/blood-2012-07-441832 [DOI] [PubMed] [Google Scholar]
- 104.Thiery J, Keefe D, Boulant S, Boucrot E, Walch M, Martinvalet D, et al. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol. 2011;12:770–7. 10.1038/ni.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375–80. 10.1084/jem.188.12.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Montel AH, Bochan MR, Hobbs JA, Lynch DH, Brahmi Z. Fas involvement in cytotoxicity mediated by human NK cells. Cell Immunol. 1995;166:236–46. 10.1006/cimm.1995.9974 [DOI] [PubMed] [Google Scholar]
- 107.Risso V, Lafont E, Le Gallo M. Therapeutic approaches targeting CD95L/CD95 signaling in cancer and autoimmune diseases. Cell Death Dis. 2022;13:1–32. 10.1038/s41419-022-04688-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee J, Dieckmann NMG, Edgar JR, Griffiths GM, Siegel RM. Fas Ligand localizes to intraluminal vesicles within NK cell cytolytic granules and is enriched at the immune synapse. Immun Inflamm Dis. 2018;6:312–21. 10.1002/iid3.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–6. 10.1038/4779 [DOI] [PubMed] [Google Scholar]
- 110.Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, et al. Tumor necrosis factor–related apoptosis-inducing ligand (trail) contributes to interferon γ–dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–70. 10.1084/jem.193.6.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. 10.1038/83416 [DOI] [PubMed] [Google Scholar]
- 112.Sheppard S, Schuster IS, Andoniou CE, Cocita C, Adejumo T, Kung SKP, et al. The murine natural cytotoxic receptor NKp46/NCR1 controls TRAIL protein expression in NK cells and ILC1s. Cell Rep. 2018;22:3385–92. 10.1016/j.celrep.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prager I, Liesche C, van Ooijen H, Urlaub D, Verron Q, Sandström N, et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J Exp Med. 2019;216:2113–27. 10.1084/jem.20181454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Monleón I, Martínez-Lorenzo MJ, Monteagudo L, Lasierra P, Taulés M, Iturralde M, et al. Differential secretion of fas ligand- or APO2 Ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167:6736–44. 10.4049/jimmunol.167.12.6736 [DOI] [PubMed] [Google Scholar]
- 115.Reefman E, Kay JG, Wood SM, Offenhäuser C, Brown DL, Roy S, et al. Cytokine secretion is distinct from secretion of cytotoxic granules in NK cells. J Immunol Balt Md 1950. 2010;184:4852–62. [DOI] [PubMed] [Google Scholar]
- 116.Sanderson NSR, Puntel M, Kroeger KM, Bondale NS, Swerdlow M, Iranmanesh N, et al. Cytotoxic immunological synapses do not restrict the action of interferon-γ to antigenic target cells. Proc Natl Acad Sci. 2012;109:7835–40. 10.1073/pnas.1116058109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharm Sin. 2008;29:1275–88. 10.1111/j.1745-7254.2008.00889.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847. 10.3389/fimmu.2018.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Almishri W, Santodomingo-Garzon T, Le T, Stack D, Mody CH, Swain MG. TNFα Augments Cytokine-Induced NK Cell IFNγ Production through TNFR2. J Innate Immun. 2016;8:617–29. 10.1159/000448077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bhat P, Leggatt G, Waterhouse N, Frazer IH. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017;8:e2836. 10.1038/cddis.2017.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verron Q, Forslund E, Brandt L, Leino M, Frisk TW, Olofsson PE, et al. NK cells integrate signals over large areas when building immune synapses but require local stimuli for degranulation. Sci Signal. 2021;14:eabe2740. 10.1126/scisignal.abe2740 [DOI] [PubMed] [Google Scholar]
- 122.Tang JJJ, Sung AP, Guglielmo MJ, Navarrete-Galvan L, Redelman D, Smith-Gagen J, et al. Natural Killer (NK) Cell Expression of CD2 as a Predictor of Serial Antibody-Dependent. Cell-Mediated Cytotox (ADCC) Antibodies Basel Switz. 2020;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deaglio S, Capobianco A, Calì A, Bellora F, Alberti F, Righi L, et al. Structural, functional, and tissue distribution analysis of human transferrin receptor-2 by murine monoclonal antibodies and a polyclonal antiserum. Blood. 2002;100:3782–9. 10.1182/blood-2002-01-0076 [DOI] [PubMed] [Google Scholar]
- 124.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. 10.1182/blood.V99.3.754 [DOI] [PubMed] [Google Scholar]
- 125.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol J Am Soc Clin Oncol. 2008;26:1789–96. 10.1200/JCO.2007.14.8957 [DOI] [PubMed] [Google Scholar]
- 126.Fan X, Yuan Z, Zhao Y, Xiong W, Hsiao HC, Pare R, et al. Impairment of IgG Fc functions promotes tumor progression and suppresses NK cell antitumor actions. Commun Biol. 2022;5:1–14. 10.1038/s42003-022-03931-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muntasell A, Rojo F, Servitja S, Rubio-Perez C, Cabo M, Tamborero D, et al. NK cell infiltrates and HLA class I expression in primary HER2+ breast cancer predict and uncouple pathological response and disease-free survival. Clin Cancer Res. 2019;25:1535–45. 10.1158/1078-0432.CCR-18-2365 [DOI] [PubMed] [Google Scholar]
- 128.Klanova M, Oestergaard MZ, Trněný M, Hiddemann W, Marcus R, Sehn LH, et al. Prognostic impact of natural killer cell count in follicular lymphoma and diffuse large B-cell lymphoma patients treated with immunochemotherapy. Clin Cancer Res J Am Assoc Cancer Res. 2019;25:4634–43. 10.1158/1078-0432.CCR-18-3270 [DOI] [PubMed] [Google Scholar]
- 129.Choi PJ, Mitchison TJ. Imaging burst kinetics and spatial coordination during serial killing by single natural killer cells. Proc Natl Acad Sci. 2013;110:6488–93. 10.1073/pnas.1221312110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gwalani LA, Orange JS. Single degranulations in NK cells can mediate target cell killing. J Immunol Balt Md 1950. 2018;200:3231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Srpan K, Ambrose A, Karampatzakis A, Saeed M, Cartwright ANR, Guldevall K, et al. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J Cell Biol. 2018;217:3267–83. 10.1083/jcb.201712085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu Y, Huang B, Shi J. Fas ligand and lytic granule differentially control cytotoxic dynamics of natural killer cell against cancer target. Oncotarget. 2016;7:47163–72. 10.18632/oncotarget.9980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anft M, Netter P, Urlaub D, Prager I, Schaffner S, Watzl C. NK cell detachment from target cells is regulated by successful cytotoxicity and influences cytokine production. Cell Mol Immunol. 2020;17:347–55. 10.1038/s41423-019-0277-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lajoie L, Congy-Jolivet N, Bolzec A, Gouilleux-Gruart V, Sicard E, Sung HC, et al. ADAM17-mediated shedding of FcγRIIIA on human NK cells: identification of the cleavage site and relationship with activation. J Immunol Balt Md 1950. 2014;192:741–51. [DOI] [PubMed] [Google Scholar]
- 135.Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood. 2013;121:3599–608. 10.1182/blood-2012-04-425397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mishra HK, Pore N, Michelotti EF, Walcheck B. Anti-ADAM17 monoclonal antibody MEDI3622 increases IFNγ production by human NK cells in the presence of antibody-bound tumor cells. Cancer Immunol Immunother CII. 2018;67:1407–16. 10.1007/s00262-018-2193-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Krzywinska E, Allende-Vega N, Cornillon A, Vo DN, Cayrefourcq L, Panabieres C, et al. Identification of Anti-tumor Cells Carrying Natural Killer (NK) Cell Antigens in Patients With Hematological Cancers. EBioMedicine. 2015;2:1364–76. 10.1016/j.ebiom.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Krzywinska E, Cornillon A, Allende-Vega N, Vo DN, Rene C, Lu ZY, et al. CD45 Isoform Profile Identifies Natural Killer (NK) Subsets with Differential Activity. PloS One. 2016;11:e0150434. 10.1371/journal.pone.0150434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vo DN, Alexia C, Allende-Vega N, Morschhauser F, Houot R, Menard C, et al. NK cell activation and recovery of NK cell subsets in lymphoma patients after obinutuzumab and lenalidomide treatment. Oncoimmunology. 2018;7:e1409322. 10.1080/2162402X.2017.1409322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vo DN, Constantinides M, Allende-Vega N, Alexia C, Cartron G, Villalba M. Dissecting the NK cell population in hematological cancers confirms the presence of tumor cells and their impact on NK population function. Vaccines. 2020;8:727. 10.3390/vaccines8040727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Soma L, Wu D, Chen X, Edlefsen K, Fromm JR, Wood B. Apparent CD19 expression by natural killer cells: a potential confounder for minimal residual disease detection by flow cytometry in B lymphoblastic leukemia. Cytom B Clin Cytom. 2015;88:145–7. 10.1002/cytob.21179 [DOI] [PubMed] [Google Scholar]
- 142.Hasim MS, Marotel M, Hodgins JJ, Vulpis E, Makinson OJ, Asif S, et al. When killers become thieves: Trogocytosed PD-1 inhibits NK cells in cancer. Sci Adv. 2022;8:eabj3286. 10.1126/sciadv.abj3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, et al. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26:1423–33. 10.1038/sj.emboj.7601570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vo DN, Leventoux N, Campos-Mora M, Gimenez S, Corbeau P, Villalba M. NK cells acquire CCR5 and CXCR4 by trogocytosis in people living with HIV-1. Vaccines. 2022;10:688. 10.3390/vaccines10050688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nakayama M, Takeda K, Kawano M, Takai T, Ishii N, Ogasawara K. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc Natl Acad Sci USA. 2011;108:18360–5. 10.1073/pnas.1110584108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suzuki E, Kataoka TR, Hirata M, Kawaguchi K, Nishie M, Haga H, et al. Trogocytosis-mediated expression of HER2 on immune cells may be associated with a pathological complete response to trastuzumab-based primary systemic therapy in HER2-overexpressing breast cancer patients. BMC Cancer. 2015;15:39. 10.1186/s12885-015-1041-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:583084. 10.3389/fimmu.2020.583084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gao J, Liang Y, Wang L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol. 2022;13:888713. 10.3389/fimmu.2022.888713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mattiola I, Pesant M, Tentorio PF, Molgora M, Marcenaro E, Lugli E, et al. Priming of human resting NK cells by autologous M1 macrophages via the engagement of IL-1β, IFN-β, and IL-15 pathways. J Immunol. 2015;195:2818–28. 10.4049/jimmunol.1500325 [DOI] [PubMed] [Google Scholar]
- 150.Zhou Z, Zhang C, Zhang J, Tian Z. Macrophages help NK cells to attack tumor cells by stimulatory NKG2D ligand but protect themselves from NK killing by inhibitory ligand Qa-1. Zimmer J, editor. PLoS ONE. 2012 May 18;7:e36928. [DOI] [PMC free article] [PubMed]
- 151.De Groen RA, Boltjes A, Hou J, Liu B, McPhee F, Friborg J, et al. IFN‐λ‐mediated IL‐12 production in macrophages induces IFN‐γ production in human NK cells. Eur J Immunol. 2015;45:250–9. 10.1002/eji.201444903 [DOI] [PubMed] [Google Scholar]
- 152.Chiba S, Ikushima H, Ueki H, Yanai H, Kimura Y, Hangai S, et al. Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. eLife. 2014;3:e04177. 10.7554/eLife.04177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nuñez SY, Ziblat A, Secchiari F, Torres NI, Sierra JM, Raffo Iraolagoitia XL, et al. Human M2 macrophages limit NK cell effector functions through secretion of TGF-β and engagement of CD85j. J Immunol. 2018;200:1008–15. 10.4049/jimmunol.1700737 [DOI] [PubMed] [Google Scholar]
- 154.Peng LS, Zhang JY, Teng YS, Zhao YL, Wang TT, Mao FY, et al. Tumor-associated monocytes/macrophages impair NK-cell function via TGFβ1 in human gastric cancer. Cancer Immunol Res. 2017;5:248–56. 10.1158/2326-6066.CIR-16-0152 [DOI] [PubMed] [Google Scholar]
- 155.Krneta T, Gillgrass A, Poznanski S, Chew M, Lee AJ, Kolb M, et al. M2-polarized and tumor-associated macrophages alter NK cell phenotype and function in a contact-dependent manner. J Leukoc Biol. 2017;101:285–95. 10.1189/jlb.3A1215-552R [DOI] [PubMed] [Google Scholar]
- 156.Klose R, Krzywinska E, Castells M, Gotthardt D, Putz EM, Kantari-Mimoun C, et al. Targeting VEGF-A in myeloid cells enhances natural killer cell responses to chemotherapy and ameliorates cachexia. Nat Commun. 2016;7:12528. 10.1038/ncomms12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gallazzi M, Baci D, Mortara L, Bosi A, Buono G, Naselli A, et al. Prostate cancer peripheral blood nk cells show enhanced CD9, CD49a, CXCR4, CXCL8, MMP-9 production and secrete monocyte-recruiting and polarizing factors. Front Immunol. 2021;11:586126. 10.3389/fimmu.2020.586126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–52. 10.1016/j.immuni.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128:4654–68. 10.1172/JCI99317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. 10.1002/hep.23054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β1. J Immunol. 2009;182:240–9. 10.4049/jimmunol.182.1.240 [DOI] [PubMed] [Google Scholar]
- 163.Stiff A, Trikha P, Mundy-Bosse B, McMichael E, Mace TA, Benner B, et al. Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing Fc receptor-mediated natural killer cell function. Clin Cancer Res J Am Assoc Cancer Res. 2018;24:1891–904. 10.1158/1078-0432.CCR-17-0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–9. 10.1182/blood-2008-03-143776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, et al. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. 10.1038/7403 [DOI] [PubMed] [Google Scholar]
- 166.Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–1037.e14. 10.1016/j.cell.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Allen F, Bobanga ID, Rauhe P, Barkauskas D, Teich N, Tong C, et al. CCL3 augments tumor rejection and enhances CD8+ T cell infiltration through NK and CD103+ dendritic cell recruitment via IFNγ. Oncoimmunology. 2018;7:e1393598. 10.1080/2162402X.2017.1393598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, et al. A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nat Med. 2018;24:1178–91. 10.1038/s41591-018-0085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Holmes TD, Wilson EB, Black EVI, Benest AV, Vaz C, Tan B, et al. Licensed human natural killer cells aid dendritic cell maturation via TNFSF14/LIGHT. Proc Natl Acad Sci USA. 2014;111:E5688–5696. 10.1073/pnas.1411072112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vitale M, Chiesa MD, Carlomagno S, Pende D, Aricò M, Moretta L, et al. NK-dependent DC maturation is mediated by TNFα and IFNγ released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–71. 10.1182/blood-2004-10-4035 [DOI] [PubMed] [Google Scholar]
- 171.Russick J, Joubert PE, Gillard-Bocquet M, Torset C, Meylan M, Petitprez F, et al. Natural killer cells in the human lung tumor microenvironment display immune inhibitory functions. J Immunother Cancer. 2020;8:e001054. 10.1136/jitc-2020-001054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee SC, Srivastava RM, López-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK):dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. 2011;50:248–54. 10.1007/s12026-011-8231-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kijima M, Yamaguchi T, Ishifune C, Maekawa Y, Koyanagi A, Yagita H, et al. Dendritic cell-mediated NK cell activation is controlled by Jagged2–Notch interaction. Proc Natl Acad Sci. 2008;105:7010–5. 10.1073/pnas.0709919105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Perez-Martinez A, Iyengar R, Gan K, Chotsampancharoen T, Rooney B, Holladay M, et al. Blood dendritic cells suppress NK cell function and increase the risk of leukemia relapse after hematopoietic cell transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2011;17:598–607. 10.1016/j.bbmt.2010.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol. 2019;9:1146. 10.3389/fonc.2019.01146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ogura K, Sato-Matsushita M, Yamamoto S, Hori T, Sasahara M, Iwakura Y, et al. NK cells control tumor-promoting function of neutrophils in mice. Cancer Immunol Res. 2018;6:348–57. 10.1158/2326-6066.CIR-17-0204 [DOI] [PubMed] [Google Scholar]
- 177.Schäkel K, Von Kietzell M, Hänsel A, Ebling A, Schulze L, Haase M, et al. Human 6-Sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity. 2006;24:767–77. 10.1016/j.immuni.2006.03.020 [DOI] [PubMed] [Google Scholar]
- 178.Costantini C, Calzetti F, Perbellini O, Micheletti A, Scarponi C, Lonardi S, et al. Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFN{gamma}: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117:1677–86. 10.1182/blood-2010-06-287243 [DOI] [PubMed] [Google Scholar]
- 179.Sun R, Xiong Y, Liu H, Gao C, Su L, Weng J, et al. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis. Transl Oncol. 2020;13:100825. 10.1016/j.tranon.2020.100825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Valayer A, Brea D, Lajoie L, Avezard L, Combes-Soia L, Labas V, et al. Neutrophils can disarm NK cell response through cleavage of NKp46. J Leukoc Biol. 2017;101:253–9. 10.1189/jlb.3AB0316-140RR [DOI] [PubMed] [Google Scholar]
- 181.Godfrey DI, Koay HF, McCluskey J, Gherardin NA. The biology and functional importance of MAIT cells. Nat Immunol. 2019;20:1110–28. 10.1038/s41590-019-0444-8 [DOI] [PubMed] [Google Scholar]
- 182.Petley EV, Koay HF, Henderson MA, Sek K, Todd KL, Keam SP, et al. MAIT cells regulate NK cell-mediated tumor immunity. Nat Commun. 2021;12:4746. 10.1038/s41467-021-25009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Speiser DE, Chijioke O, Schaeuble K, Münz C. CD4+ T cells in cancer. Nat Cancer. 2023;4:317–29. 10.1038/s43018-023-00521-2 [DOI] [PubMed] [Google Scholar]
- 184.Nakayama M, Takeda K, Kawano M, Takai T, Ishii N, Ogasawara K. Natural killer (NK)–dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4 + T cells. Proc Natl Acad Sci. 2011;108:18360–5. 10.1073/pnas.1110584108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Adam C, King S, Allgeier T, Braumüller H, Lüking C, Mysliwietz J, et al. DC-NK cell cross talk as a novel CD4+ T-cell–independent pathway for antitumor CTL induction. Blood. 2005;106:338–44. 10.1182/blood-2004-09-3775 [DOI] [PubMed] [Google Scholar]
- 186.Brillard E, Pallandre JR, Chalmers D, Ryffel B, Radlovic A, Seilles E, et al. Natural killer cells prevent CD28-mediated Foxp3 transcription in CD4+CD25– T lymphocytes. Exp Hematol. 2007;35:416–25. 10.1016/j.exphem.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 187.Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat Immunol. 2004;5:1260–5. 10.1038/ni1138 [DOI] [PubMed] [Google Scholar]
- 188.Prins RM, Vo DD, Khan-Farooqi H, Yang MY, Soto H, Economou JS, et al. NK and CD4 cells collaborate to protect against melanoma tumor formation in the brain. J Immunol Balt Md 1950. 2006;177:8448–55. [DOI] [PubMed] [Google Scholar]
- 189.Wang Z, Chimenti MS, Strouse C, Weiner GJ. T cells, particularly activated CD4+ cells, maintain anti-CD20-mediated NK cell viability and antibody dependent cellular cytotoxicity. Cancer Immunol Immunother. 2022;71:237–49. 10.1007/s00262-021-02976-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity1. J Immunol. 1999;163:5211–8. 10.4049/jimmunol.163.10.5211 [DOI] [PubMed] [Google Scholar]
- 191.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res J Am Assoc Cancer Res. 2003;9:606–12. [PubMed] [Google Scholar]
- 192.Trzonkowski P, Szmit E, Myśliwska J, Myśliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of CTL and NK cells in humans—impact of immunosenescence. Clin Immunol. 2006;119:307–16. 10.1016/j.clim.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 193.Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor–β–dependent manner. J Exp Med. 2005;202:1075–85. 10.1084/jem.20051511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wong JL, Berk E, Edwards RP, Kalinski P. IL-18–primed helper NK cells collaborate with dendritic cells to promote recruitment of effector CD8+ T cells to the tumor microenvironment. Cancer Res. 2013;73:4653–62. 10.1158/0008-5472.CAN-12-4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Iraolagoitia XLR, Spallanzani RG, Torres NI, Araya RE, Ziblat A, Domaica CI, et al. NK cells restrain spontaneous antitumor CD8+ T cell priming through PD-1/PD-L1 interactions with dendritic cells. J Immunol. 2016;197:953–61. 10.4049/jimmunol.1502291 [DOI] [PubMed] [Google Scholar]
- 196.Bai L, Peng H, Hao X, Tang L, Sun C, Zheng M, et al. CD8+ T cells promote maturation of liver‐resident NK cells through the CD70‐CD27 axis. Hepatology. 2019;70:1804–15. 10.1002/hep.30757 [DOI] [PubMed] [Google Scholar]
- 197.Somersalo K, Carpén O, Saksela E. Stimulated natural killer cells secrete factors with chemotactic activity, including NAP‐1/IL‐8, which supports VLA‐4‐ and VLA‐5‐mediated migration of T lymphocytes. Eur J Immunol. 1994;24:2957–65. 10.1002/eji.1830241206 [DOI] [PubMed] [Google Scholar]
- 198.Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006;66:517–26. 10.1158/0008-5472.CAN-05-2429 [DOI] [PubMed] [Google Scholar]
- 199.Shanker A, Verdeil G, Buferne M, Inderberg-Suso EM, Puthier D, Joly F, et al. CD8 T cell help for innate antitumor immunity. J Immunol Balt Md 1950. 2007;179:6651–62. [DOI] [PubMed] [Google Scholar]
- 200.Villalba M, Alexia C, Bellin-Robert A, Fayd’herbe de Maudave A, Gitenay D. Non-genetically improving the natural cytotoxicity of natural killer (NK) cells. Front Immunol. 2019;10:3026. 10.3389/fimmu.2019.03026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The relevant information is available from the corresponding authors upon reasonable request.