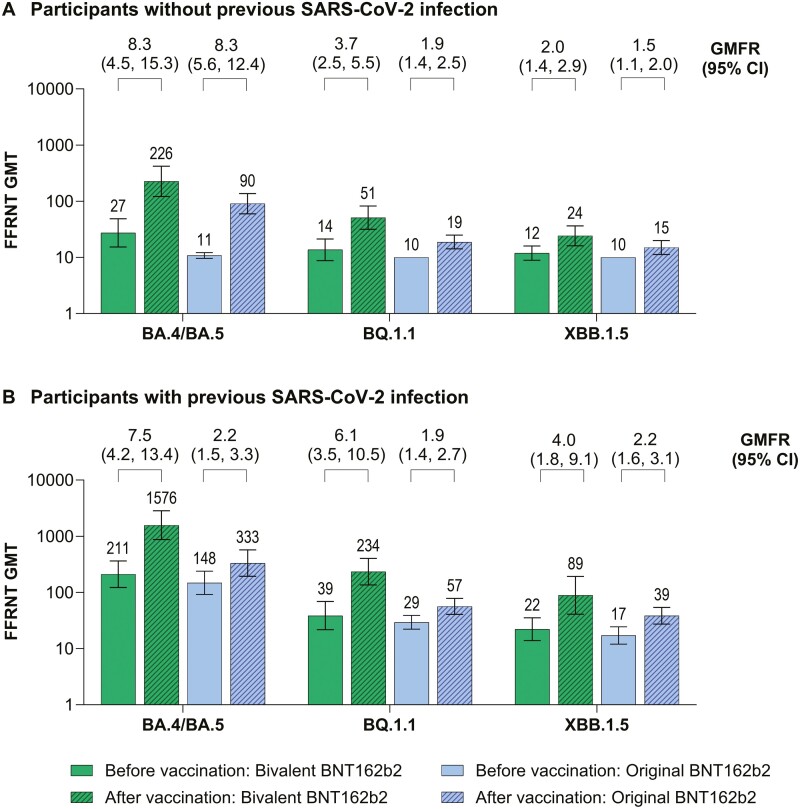
Figure 2.
SARS-CoV-2 fluorescent focus reduction neutralization test assay results before and 1 month after vaccination with bivalent BNT162b2 (dose 4) or original BNT162b2 (dose 3) for Omicron BA.4/BA.5, BQ.1.1, and XBB.1.5 strains in children 6 months to <5 years old (A) without previous SARS-CoV-2 infection and (B) with previous SARS-CoV-2 infection. Shown are GMTs before and 1 month after vaccination and associated GMFRs. Data are for the evaluable SARS-CoV-2 variant immunogenicity subset and include participants with (n = 9–10) or without (n = 17–20) evidence of previous SARS-CoV-2 infection (Supplementary Table 2). This subset included 30 participants who had sufficient blood sample volume for additional testing among the first 24 and 36 participants assigned in the 6 months to <2 years age group and 2 years to <5 years age group, respectively, and had ≥1 valid and determinate immunogenicity result within the required window. GMTs are shown immediately above the bars and GMFRs and 95% CIs from before to 1 month after vaccination are shown in brackets above the bars. GMTs, GMFRs, and associated 95% CIs were calculated by exponentiating the mean logarithm of the titers (GMTs) or fold rises (GMFRs) and the corresponding CIs (based on the Student’s t distribution); assay results below the LLOQ were set to 0.5 × LLOQ. Data for original BNT162b2 are in participants from the original pediatric study (NCT04816643) who were matched by age, baseline SARS-CoV-2 status, and time since previous BNT162b2 dose (see Supplementary Table 5 for demographic details for these participants and those of the evaluable SARS-CoV-2 variant immunogenicity population of the current study). The definition of with or without previous SARS-CoV-2 infection is provided in the Supplementary Appendix. FFRNT, fluorescent focus reduction neutralization test; GMT, geometric mean titer; GMFR, geometric mean-fold rise.