Figure 1.
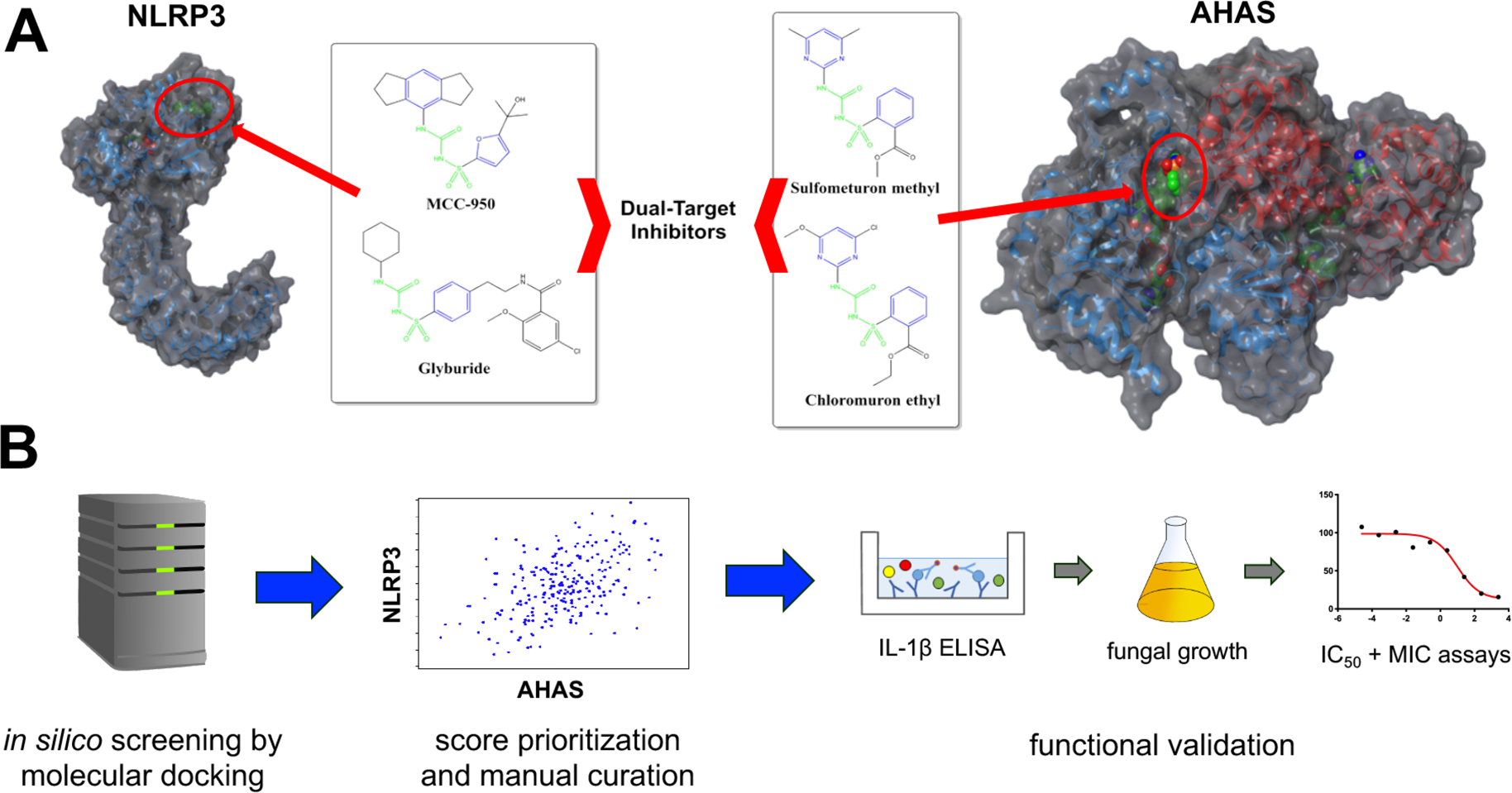
Approach used to identify dual-target inhibitors. (A) Established inhibitors of the NLRP3 inflammasome include sulfonylureas MCC950 and hypoglycemic agents such as glyburide. Inhibitors of yeast acetohydroxyacid synthase (AHAS) include a series of herbicidal sulfonylurea agents including chlorimuron ethyl and sulfometuron. Identical chemical features in both sets of compounds are highlighted in green and similar chemical features are highlighted in blue. The predicted NLRP3 and known AHAS binding sites are indicated by red circles. (B) The Maybridge screening collection of over 53000 compounds was used to perform in silico molecular docking for both NLRP3 and AHAS. Compounds scoring in the top 1% for each target were cross-referenced and common hits were ordered for experimental validation. Generally, compounds were first tested for their capacity to inhibit IL-1β release in THP1 cells stimulated with the inflammasome inducers LPS and ATP. Compounds showing good efficacy were tested for their capacity to inhibit C. albicans growth. Compounds possessing both anti-inflammatory and antifungal properties at a dose of 50 μM were further characterized by establishing inhibitory concentration 50 (IC50) and modified minimal inhibitory concentration (MIC) values.
