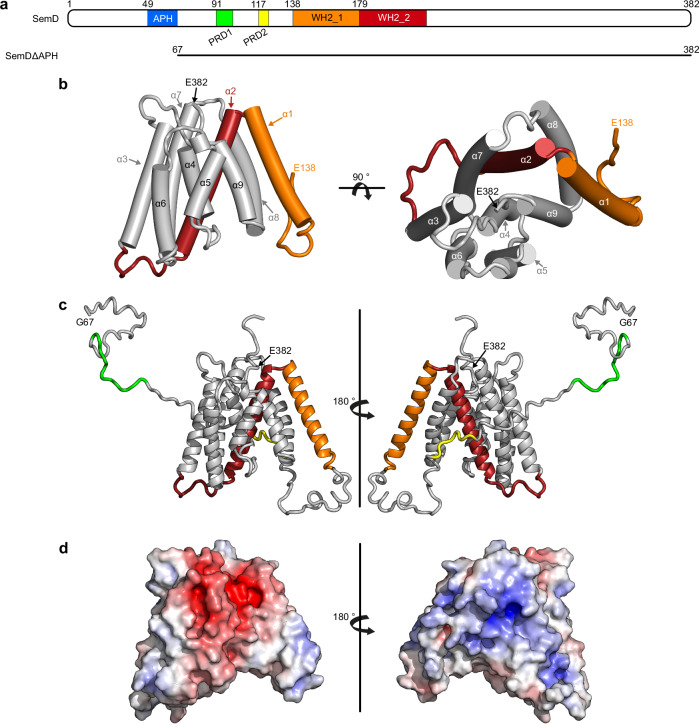
Fig. 1. Crystal structure of SemDΔAPH.
a Schematic representation of the primary structure of SemD, containing an APH49-66, two proline-rich domains (PRD191-100, PRD2117-122) and two WH2 domains (WH2_1138-178, WH2_2179-216). SemDΔAPH67-382 is represented as a black bar. b The structure of SemDΔAPH as resolved by X-ray crystallography. The helices are depicted as cylinders and numbered from 1 to 9, starting at the N-terminus (α1- α9). In accordance with the colour code in a, the WH2_1 and WH2_2 are depicted in orange and red, respectively. The N-terminal E138 and the C-terminal E382 (both marked by black arrows) represent the first and last amino acids visible in the electron density. Right panel: 90° rotation. c SAXS best-fit CORAL model (χ2 value of 1.197), based on the SemDΔAPH crystal structure, and including the added flexible tails (further models are shown in Supplementary Fig. 1h). PRD1 and PRD2 are coloured in green and yellow, respectively. Right panel: 180° rotation. G67 is the N-terminal amino acid, while E382, the last C-terminal residue of SemD, is followed by the C-terminal 10x-His-Tag. d Electrostatic surface representation of SemDΔAPH highlighting the negatively (red) and positively (blue) charged patches. Right panel: 180° rotation.