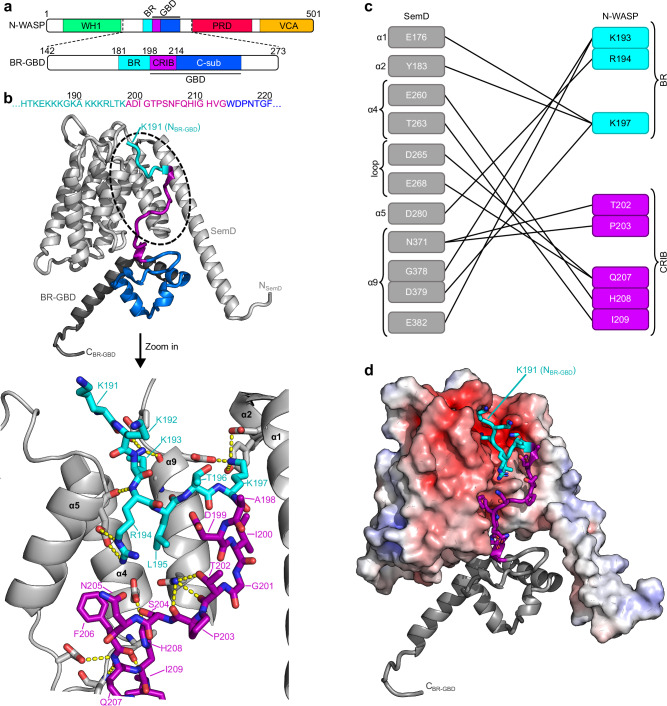
Fig. 2. SemD engages with BR-GBD in a Cdc42GTP-mimicking manner.
a Schematic representation of the N-WASP primary sequence. BR-GBD142-273 was used for complex formation with SemDΔAPH. It contains the BR181-197 domain (basic region, cyan), the CRIB198-213 domain (Cdc42/Rac interactive binding motif, magenta) and the C-sub214-250 domain (blue). b The structure of SemDΔAPH in complex with BR-GBD as resolved by X-ray crystallography, shown in cartoon representation. SemDΔAPH is shown in light grey, BR-GBD is coloured in dark grey with the BR domain in cyan, the CRIB domain in magenta and C-sub domain in blue. The zoom in shows details of the binding of SemDΔAPH to BR-GBD. Important residues of SemDΔAPH and BR-GBD are shown in stick representation, while the rest of SemDΔAPH is shown as cartoon. Interactions (<3.5 Å) are shown by the yellow dashes. c Schematic representation of the detailed interactions between SemDΔAPH and BR-GBD. d Electrostatic representation of SemDΔAPH, highlighting the negatively charged patch in red and positively charged surface areas in blue. BR-GBD is coloured in dark grey (cartoon) with the BR domain in cyan and the CRIB domain in magenta, both depicted with stick residues.