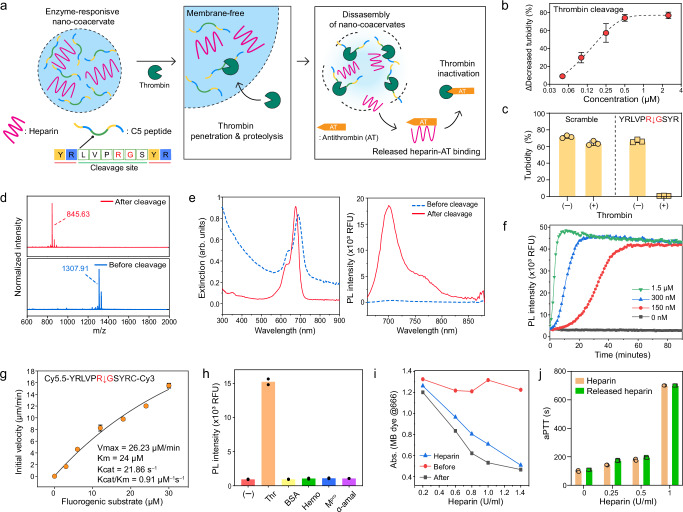
Fig. 2. Thrombin-responsive nano-coacervates.
a Schematic illustration of heparin release through disassembly of nano-coacervates driven by thrombin proteolysis. The released heparin binds antithrombin which induces thrombin inactivation. b Decreased turbidity of nano-coacervates as a function of thrombin concentrations. c Turbidity changes of C5- and C6-based nano-coacervates with and without thrombin (5 µM). d MALDI-MOF data before and after thrombin cleavage, confirming the mass peak of the parent (Mw: 1307.91) and its fragment (Mw: 845.63). The N-terminus of C5 was acetylated. e UV-vis and PL spectra of C7-encapsulated nano-coacervates before and after thrombin cleavage. The quenched PL signal of sulfo-Cy5.5 dyes activated as a function of nano-coacervates’ disassembly. f Time-dependent PL670 nm changes driven by thrombin cleavage. g kcat/KM determination for C5 peptide cleavage driven by thrombin proteolysis. The thrombin (20 nM) was incubated with a fluorogenic substrate ([S]0 = 0–30 µM, sequence shown on top in the panel box), and the product concentration at 30 min was used. Data was fit to the Michaelis–Menten equation (see Supplementary information 2.8). h Specificity test using different biological proteins including thrombin (Thr), bovine serum albumin (BSA), hemoglobin (Hemo), main protease of SARS-CoV-19 (Mpro), and α-amylase (50 U/ml). A sample without any proteins is referred to as a negative control. i Decreased absorbance of MB dye before and after the addition of thrombin. The disassembly of nano-coacervates released heparin, leading to a decrease in the absorbance of MB dye while intact nano-coacervates showed a negligible change in absorbance (Supplementary Fig. 17). j aPTT test of heparin and released heparin from the disassembly of nano-coacervates. Data in (f) and (h) represent the mean value of two independent samples. Data in (b), (c), (g), and (j) represent mean ± SD (n = 3).