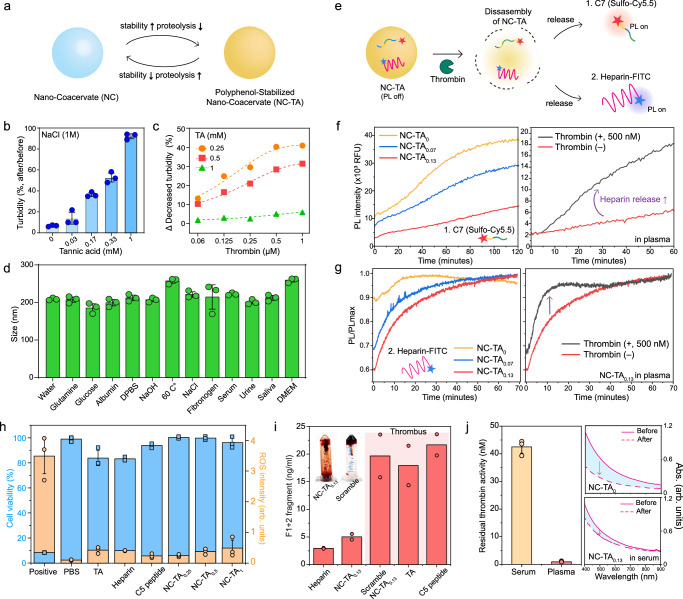
Fig. 4. Enhanced stability of NC-TAs and their proteolytic efficiency.
a Schematic illustration of a trade-off between stability and proteolysis-based disassembly of NC-TAs. NC-TAs increased stability (b) in NaCl as a function of TA encapsulations while reducing their proteolytic efficiencies (c). Thrombin was unable to dissociate NC-TA1. d Size profiles of NC-TA0.13 in different biological environments. e Schematic illustration of monitoring either C7 peptide (f) or heparin-FITC (g) during disassembly of NC-TAs by thrombin. The left panels in (f, g) show a decrease in the PL activation rate of NC-TAs compared to nano-coacervates (i.e., NC-TA0) due to improved stability in 50% human plasma. The right panels in (f, g) illustrate the addition of thrombin rapidly activates the PL intensity of C7 peptide or heparin-FITC, indicating proteolysis-driven heparin release. h Cell viability (blue) and ROS intensity (orange) of HUVEC incubating with PBS, TA, heparin, C5 peptide, and NC-TAs, respectively. i Prothrombin F1 + 2 peptide concentrations of NC-TA0.13 TA, C5 peptide, and NC-TA0.13 made of scramble peptide (i.e., C6) from whole human blood incubation. The inserted photo shows a strong blood clot from blood anticoagulation from NC-TA0.13 (left) and scramble NC-TA0.13 (right). j Residual thrombin activity in human serum and plasma. Human serum shows higher residual thrombin activity comparable to 42.5 nM of alpha-thrombin. The graphs on the right panel in (j) represent absorbance changes of nano-coacervates and NC-TA0.13 before and after 1 h incubation in 50% human serum, showing higher stability of NC-TA0.13 than pristine nano-coacervates. Data in (c), (f), (g), and (i) represent the mean value of two independent samples. Data in (b), (d), (h), and (j) represent mean ± SD (n = 3).