Abstract
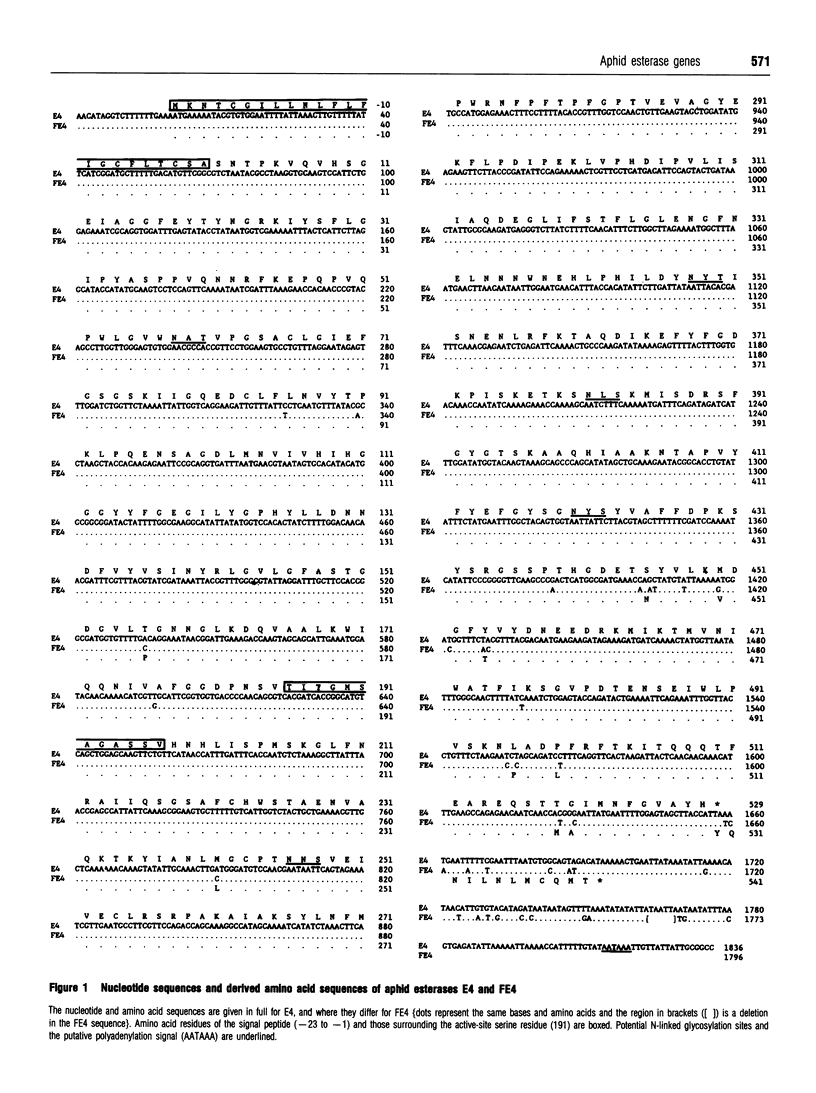
Full-length cDNA clones encoding the esterases (E4 and FE4) that confer insecticide resistance in the peach-potato aphid [Myzus persicae (Sulzer)] were isolated and characterized. The E4 cDNA contained an open reading frame of 1656 nucleotides, coding for a protein of 552 amino acids. The FE4 cDNA shared 99% identity with E4 over this region, the most important difference being a single nucleotide substitution resulting in the FE4 mRNA having an extra 36 nucleotides at the 3' end. The derived amino acid sequences for the N-terminus of E4 and FE4 were identical, with the first 23 residues being characteristic of a signal peptide and the next 40 residues being an exact match to the N-terminal sequence determined by Edman degradation of both purified proteins. The predicted molecular masses of 58.8 and 60.2 kDa for the E4 and FE4 polypeptides were consistent with those previously observed by in vitro translation of mRNA. Five potential N-linked glycosylation sites were present in both polypeptides, in accordance with earlier evidence that the native esterases are glycoproteins. Comparison of the aphid esterase protein sequences with other serine hydrolases provided evidence that their activity involves a charge-relay system with a catalytic triad the same as that found in acetylcholinesterase. Restriction mapping and sequencing of cloned genomic DNA showed that the E4 gene is spread over 4.3 kb with six introns and that the previously reported differences between the 3' ends of the E4 and FE4 genes result from single nucleotide substitutions and not gross differences in the DNA sequences.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bause E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J. 1983 Feb 1;209(2):331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P., Wells J. A. Dissecting the catalytic triad of a serine protease. Nature. 1988 Apr 7;332(6164):564–568. doi: 10.1038/332564a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collet C., Nielsen K. M., Russell R. J., Karl M., Oakeshott J. G., Richmond R. C. Molecular analysis of duplicated esterase genes in Drosophila melanogaster. Mol Biol Evol. 1990 Jan;7(1):9–28. doi: 10.1093/oxfordjournals.molbev.a040582. [DOI] [PubMed] [Google Scholar]
- Cooke P. H., Oakeshott J. G. Amino acid polymorphisms for esterase-6 in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1426–1430. doi: 10.1073/pnas.86.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. M., Devonshire A. L., Forde B. G. Molecular evidence that insecticide resistance in peach-potato aphids (Myzus persicae Sulz.) results from amplification of an esterase gene. Biochem J. 1988 Apr 1;251(1):309–312. doi: 10.1042/bj2510309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Malcolm C. A. The acetylcholinesterase gene of Anopheles stephensi. Cell Mol Neurobiol. 1991 Feb;11(1):131–141. doi: 10.1007/BF00712805. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Spierer P. The Ace locus of Drosophila melanogaster: structural gene for acetylcholinesterase with an unusual 5' leader. EMBO J. 1986 Nov;5(11):2949–2954. doi: 10.1002/j.1460-2075.1986.tb04591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzlik T. N., Abdel-Aal Y. A., Harshman L. G., Hammock B. D. Isolation and sequencing of cDNA clones coding for juvenile hormone esterase from Heliothis virescens. Evidence for a catalytic mechanism for the serine carboxylesterases different from that of the serine proteases. J Biol Chem. 1989 Jul 25;264(21):12419–12425. [PubMed] [Google Scholar]
- Korza G., Ozols J. Complete covalent structure of 60-kDa esterase isolated from 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced rabbit liver microsomes. J Biol Chem. 1988 Mar 5;263(7):3486–3495. [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen R. A. Solid-phase Edman degradation. An automatic peptide sequencer. Eur J Biochem. 1971 May 11;20(1):89–102. doi: 10.1111/j.1432-1033.1971.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Long R. M., Satoh H., Martin B. M., Kimura S., Gonzalez F. J., Pohl L. R. Rat liver carboxylesterase: cDNA cloning, sequencing, and evidence for a multigene family. Biochem Biophys Res Commun. 1988 Oct 31;156(2):866–873. doi: 10.1016/s0006-291x(88)80924-0. [DOI] [PubMed] [Google Scholar]
- MacPhee-Quigley K., Vedvick T. S., Taylor P., Taylor S. S. Profile of the disulfide bonds in acetylcholinesterase. J Biol Chem. 1986 Oct 15;261(29):13565–13570. [PubMed] [Google Scholar]
- Mouches C., Pauplin Y., Agarwal M., Lemieux L., Herzog M., Abadon M., Beyssat-Arnaouty V., Hyrien O., de Saint Vincent B. R., Georghiou G. P. Characterization of amplification core and esterase B1 gene responsible for insecticide resistance in Culex. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2574–2578. doi: 10.1073/pnas.87.7.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M., Richmond R. C., Oakeshott J. G. On the origins of esterases. Mol Biol Evol. 1988 Mar;5(2):113–119. doi: 10.1093/oxfordjournals.molbev.a040485. [DOI] [PubMed] [Google Scholar]
- Nicholls R. D., Hill A. V., Clegg J. B., Higgs D. R. Direct cloning of specific genomic DNA sequences in plasmid libraries following fragment enrichment. Nucleic Acids Res. 1985 Nov 11;13(21):7569–7578. doi: 10.1093/nar/13.21.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakeshott J. G., Collet C., Phillis R. W., Nielsen K. M., Russell R. J., Chambers G. K., Ross V., Richmond R. C. Molecular cloning and characterization of esterase-6, a serine hydrolase of Drosophila. Proc Natl Acad Sci U S A. 1987 May;84(10):3359–3363. doi: 10.1073/pnas.84.10.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovnic M., Tepperman K., Medda S., Elliott R. W., Stephenson D. A., Grant S. G., Ganschow R. E. Characterization of a murine cDNA encoding a member of the carboxylesterase multigene family. Genomics. 1991 Feb;9(2):344–354. doi: 10.1016/0888-7543(91)90263-e. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A., Murray N. E., Revel H., Blumenthal R. M., Westaway D., Reith A. D., Rigby P. W., Elhai J., Hanahan D. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 1988 Feb 25;16(4):1563–1575. doi: 10.1093/nar/16.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafferman A., Kronman C., Flashner Y., Leitner M., Grosfeld H., Ordentlich A., Gozes Y., Cohen S., Ariel N., Barak D. Mutagenesis of human acetylcholinesterase. Identification of residues involved in catalytic activity and in polypeptide folding. J Biol Chem. 1992 Sep 5;267(25):17640–17648. [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Wachter E., Machleidt W., Hofner H., Otto J. Aminopropyl glass and its p-phenylene diisothiocyanate derivative, a new support in solid-phase Edman degradation of peptides and proteins. FEBS Lett. 1973 Sep 1;35(1):97–102. doi: 10.1016/0014-5793(73)80585-x. [DOI] [PubMed] [Google Scholar]
- Ward V. K., Bonning B. C., Huang T., Shiotsuki T., Griffeth V. N., Hammock B. D. Analysis of the catalytic mechanism of juvenile hormone esterase by site-directed mutagenesis. Int J Biochem. 1992 Dec;24(12):1933–1941. doi: 10.1016/0020-711x(92)90289-d. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Abrahmsén L. Species-specific variation in signal peptide design. Implications for protein secretion in foreign hosts. FEBS Lett. 1989 Feb 27;244(2):439–446. doi: 10.1016/0014-5793(89)80579-4. [DOI] [PubMed] [Google Scholar]


