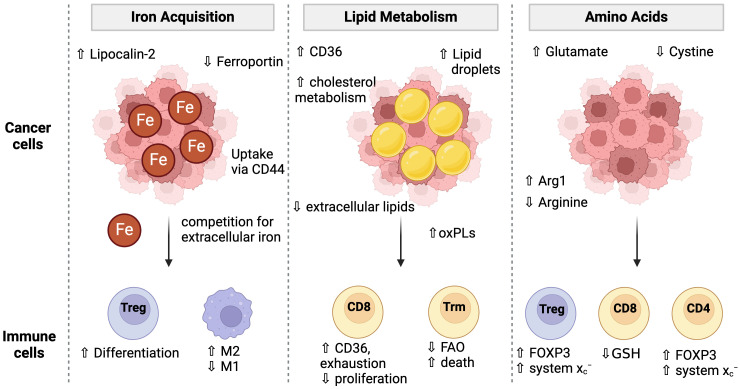
Figure 3.
Cancer cell metabolism and conditioning of the TME for immune cells. Cancer cells adopt multiple strategies to fuel their proliferation and survival including the acquisition of iron, lipids, and amino acids. These metabolic programs condition the TME to have low iron and amino acids which then impacts immune cell metabolism and ferroptosis sensitivity. Cancer cells have unique iron uptake mechanisms such as lipocalin-2 and CD44 mediated uptake while simultaneously downregulating the iron exporter, Ferroportin. Low iron conditions favor Treg differentiation over Th1 or Th17 cells and promotes M1 over M2 macrophage differentiation. Cancer cells increase lipid uptake via CD36 and lipid droplet storage. The extracellular lipid environment is altered to contain a higher proportion of oxidized phospholipids which can cause toxicity to T cells and promote CD8 T cell exhaustion. Cancer cell metabolism high in Arginase-1 (Arg1) may deplete extracellular arginine. Low arginine promotes FOXP3 expression in CD4 T cells giving them suppressive activity. Low arginine and/or cystine causes T cells to upregulate the system Xc - to increase their GSH pools. However, cancer cell mediated cystine starvation ultimately leads to low GSH levels in T cells.