Abstract
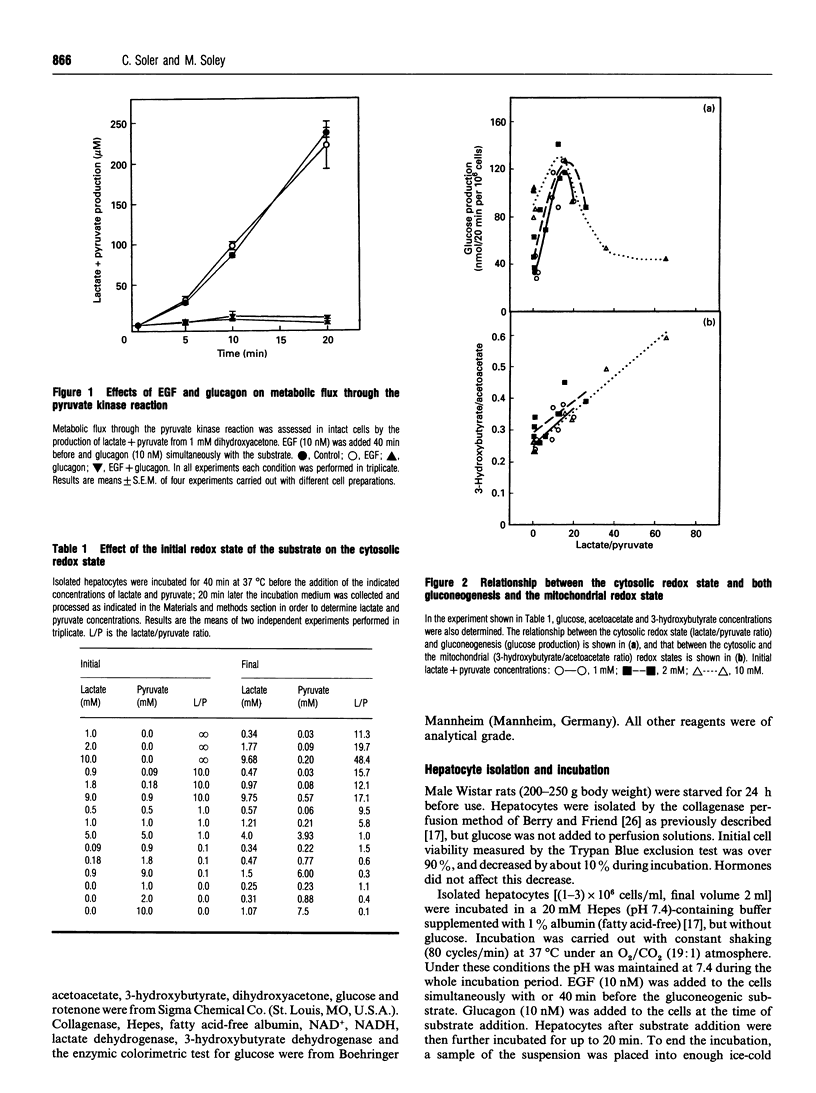
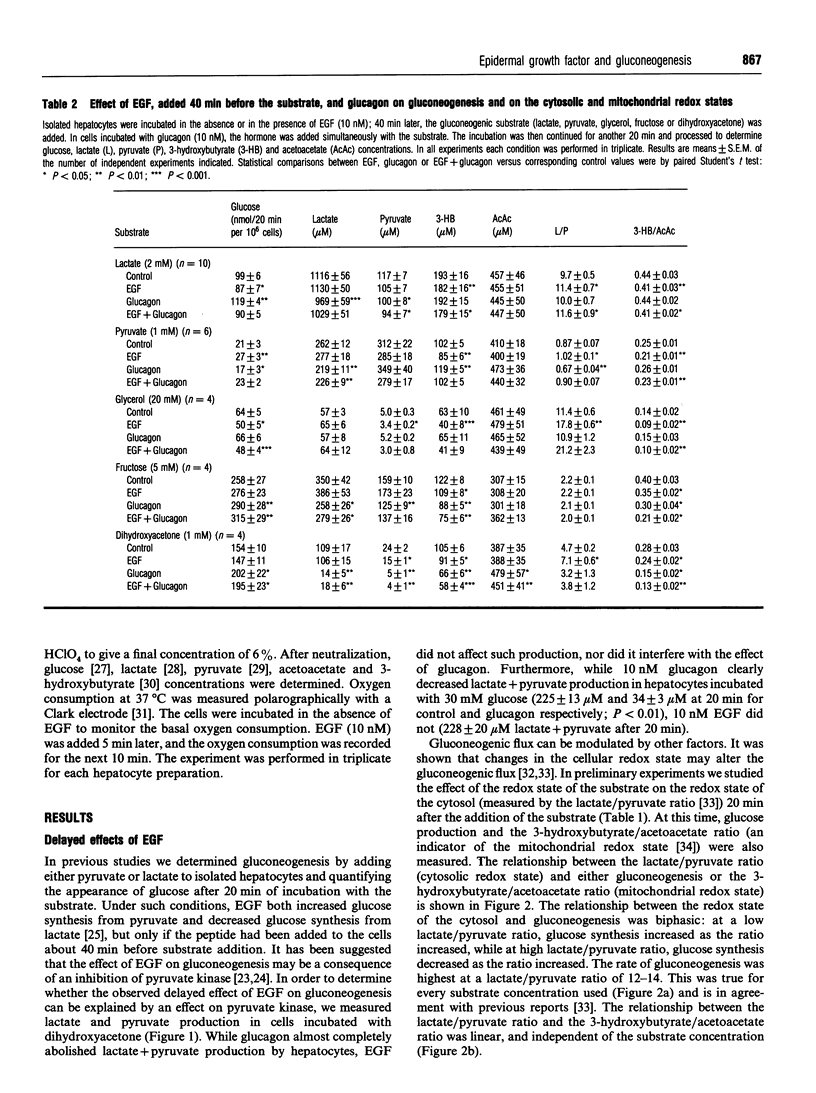
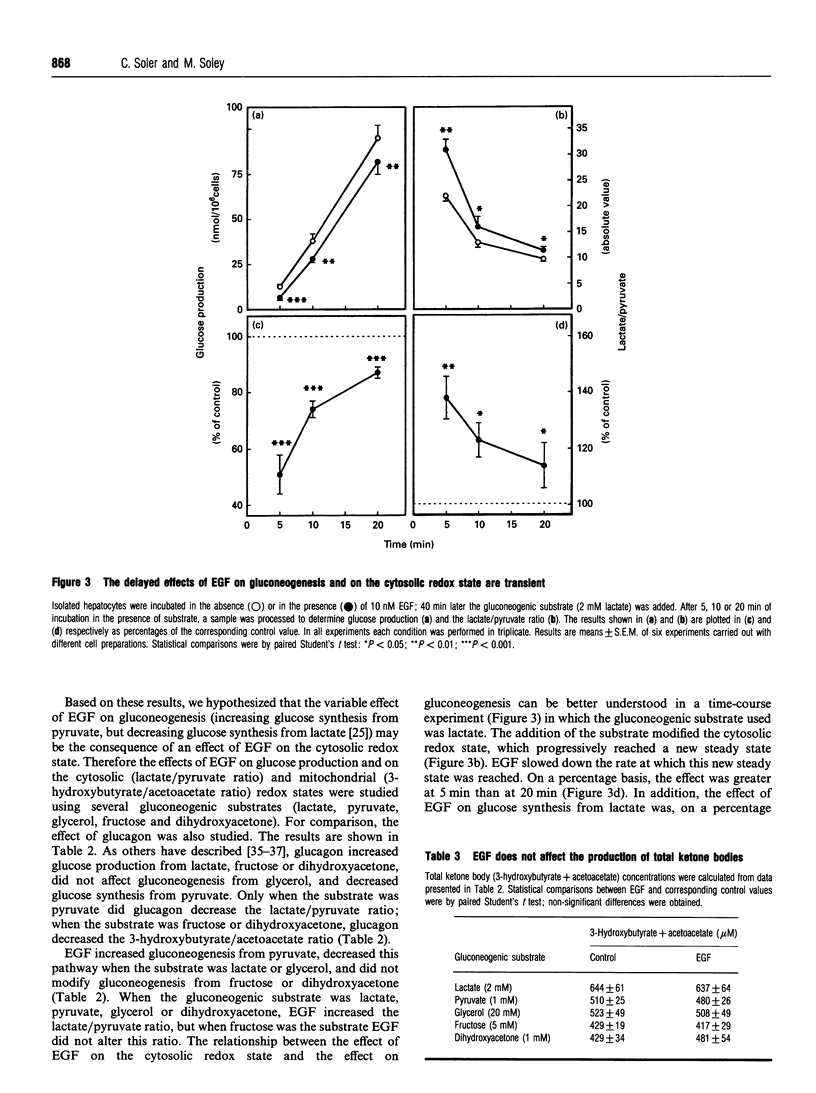
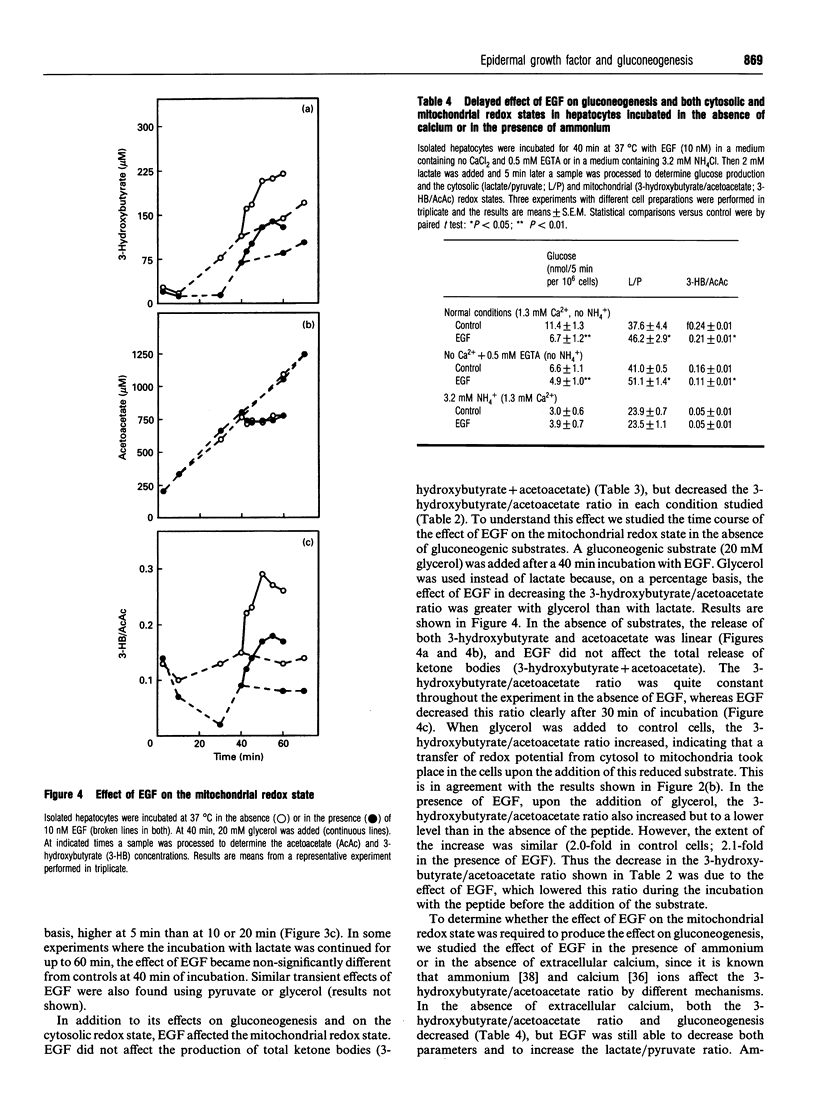
Most reports on the effects of epidermal growth factor (EGF) on gluconeogenesis have indicated that such effects depend on the substrate used and are only observable after a lag time of 30-40 min. Recently, an immediate and transient effect of EGF on glucose synthesis was described in a perfused liver system. Here we extend the study of the effect of EGF on gluconeogenesis to isolated hepatocytes from fasted rats. The delayed effect of EGF on gluconeogenesis was studied by adding the substrate 40 min after the peptide. Under these conditions EGF increased glucose synthesis from pyruvate, decreased it when the substrate was lactate or glycerol and did not modify gluconeogensis from fructose or dihydroxyacetone. EGF did not affect the metabolic flux through glycolysis, determined as the production of lactate+pyruvate from 30 mM glucose. Furthermore, EGF did not modify the metabolic flux through pyruvate kinase, determined as the production of lactate+pyruvate from 1 mM dihydroxyacetone. The differing effects of EGF on gluconeogenesis depending on the substrate used can be explained by the effects of EGF on the cytosolic redox state (measured as the lactate/pyruvate ratio). About 20 min after the addition of EGF, the mitochondrial redox state (measured as the 3-hydroxybutyrate/acetoacetate ratio) decreased. This effect of EGF was blocked by ammonium, which also abolished the effect of the peptide on gluconeogenesis. Thus the effect of EGF at the mitochondrial level appears to be necessary for its effects on gluconeogenesis. Taken together, our results indicate that the delayed effects of EGF on gluconeogenesis are secondary to the effects of the peptide at both the mitochondrial and cytosolic levels. In addition to these delayed effects, we observed that EGF rapidly and transiently stimulated glucose synthesis from lactate, decreased the cytosolic redox state and increased oxygen consumption. All of these rapid effects required the presence of extracellular calcium and disappeared in the presence of rotenone, suggesting that this rapid effect of EGF on gluconeogenesis is secondary to the stimulation of mitochondrial respiration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Meek J. The ontogeny of epidermal growth factor receptors during mouse development. Dev Biol. 1984 May;103(1):62–70. doi: 10.1016/0012-1606(84)90007-1. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F., Bouscarel B., Slaton J., Blackmore P. F., Exton J. H. Epidermal growth factor mimics insulin effects in rat hepatocytes. Biochem J. 1986 Nov 1;239(3):523–530. doi: 10.1042/bj2390523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Chowdhury M. H., Agius L. Epidermal growth factor counteracts the glycogenic effect of insulin in parenchymal hepatocyte cultures. Biochem J. 1987 Oct 15;247(2):307–314. doi: 10.1042/bj2470307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus T. H., El-Maghrabi M. R., Pilkis S. J. Modulation of the phosphorylation state of rat liver pyruvate kinase by allosteric effectors and insulin. J Biol Chem. 1979 Aug 25;254(16):7855–7864. [PubMed] [Google Scholar]
- Conricode K. M., Ochs R. S. Epidermal growth factor and 12-O-tetradecanoylphorbol 13-acetate stimulate lactate production and the pentose phosphate pathway in freshly isolated rat hepatocytes. J Biol Chem. 1990 Dec 5;265(34):20931–20937. [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. The role of calcium in the regulation of mitochondrial metabolism. Biochem Soc Trans. 1980 Jun;8(3):266–268. doi: 10.1042/bst0080266. [DOI] [PubMed] [Google Scholar]
- Diamond I., Legg A., Schneider J. A., Rozengurt E. Glycolysis in quiescent cultures of 3T3 cells. Stimulation by serum, epidermal growth factor, and insulin in intact cells and persistence of the stimulation after cell homogenization. J Biol Chem. 1978 Feb 10;253(3):866–871. [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Foster J. L., Blair J. B. Acute hormonal control of pyruvate kinase and lactate formation in the isolated rat hepatocyte. Arch Biochem Biophys. 1978 Aug;189(2):263–276. doi: 10.1016/0003-9861(78)90212-6. [DOI] [PubMed] [Google Scholar]
- González-Manchón C., Menaya J., Ayuso M. S., Parrilla R. Ca2(+)-fatty acid interaction in the control of hepatic gluconeogenesis. Biochim Biophys Acta. 1990 Mar 9;1051(3):215–220. doi: 10.1016/0167-4889(90)90125-w. [DOI] [PubMed] [Google Scholar]
- González-Manchón C., Sánchez-Ayuso M., Parrilla R. Control of gluconeogenesis: role of fatty acids in the alpha-adrenergic response. Biochim Biophys Acta. 1988 Nov 18;972(2):192–199. doi: 10.1016/0167-4889(88)90117-6. [DOI] [PubMed] [Google Scholar]
- Holland R., Hardie D. G. Both insulin and epidermal growth factor stimulate fatty acid synthesis and increase phosphorylation of acetyl-CoA carboxylase and ATP-citrate lyase in isolated hepatocytes. FEBS Lett. 1985 Feb 25;181(2):308–312. doi: 10.1016/0014-5793(85)80282-9. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Hsu C. Y., Hurwitz D. R., Mervic M., Zilberstein A. Autophosphorylation of the intracellular domain of the epidermal growth factor receptor results in different effects on its tyrosine kinase activity with various peptide substrates. Phosphorylation of peptides representing Tyr(P) sites of phospholipase C-gamma. J Biol Chem. 1991 Jan 5;266(1):603–608. [PubMed] [Google Scholar]
- Hue L., Bartrons R. Role of fructose 2,6-bisphosphate in the control by glucagon of gluconeogenesis from various precursors in isolated rat hepatocytes. Biochem J. 1984 Feb 15;218(1):165–170. doi: 10.1042/bj2180165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Crofts J. N., Auld A. M., Read L. C., Barritt G. J. Evidence that a pertussis-toxin-sensitive substrate is involved in the stimulation by epidermal growth factor and vasopressin of plasma-membrane Ca2+ inflow in hepatocytes. Biochem J. 1987 Dec 15;248(3):911–918. doi: 10.1042/bj2480911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Garrison J. C. Epidermal growth factor and angiotensin II stimulate formation of inositol 1,4,5- and inositol 1,3,4-trisphosphate in hepatocytes. Differential inhibition by pertussis toxin and phorbol 12-myristate 13-acetate. J Biol Chem. 1987 Dec 25;262(36):17285–17293. [PubMed] [Google Scholar]
- Kneer N. M., Wagner M. J., Lardy H. A. Regulation by calcium of hormonal effects on gluconeogenesis. J Biol Chem. 1979 Dec 10;254(23):12160–12168. [PubMed] [Google Scholar]
- Leverve X. M., Groen A. K., Verhoeven A. J., Tager J. M. Kinetic analysis of short-term effects of alpha-agonists on gluconeogenesis in isolated rat hepatocytes. FEBS Lett. 1985 Feb 11;181(1):43–46. doi: 10.1016/0014-5793(85)81110-8. [DOI] [PubMed] [Google Scholar]
- Leverve X. M., Verhoeven A. J., Groen A. K., Meijer A. J., Tager J. M. The malate/aspartate shuttle and pyruvate kinase as targets involved in the stimulation of gluconeogenesis by phenylephrine. Eur J Biochem. 1986 Mar 17;155(3):551–556. doi: 10.1111/j.1432-1033.1986.tb09523.x. [DOI] [PubMed] [Google Scholar]
- Mapes J. P., Harris R. A. Inhibition of gluconeogenesis and lactate formation from pyruvate by N6, O2'-dibutyryl adenosine 3':5'-monophosphate. J Biol Chem. 1976 Oct 25;251(20):6189–6196. [PubMed] [Google Scholar]
- Maswoswe S. M., Daneshmand F., Davies D. R. Metabolic effects of D-glyceraldehyde in isolated hepatocytes. Biochem J. 1986 Dec 15;240(3):771–776. doi: 10.1042/bj2400771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. A., Strain A. J., Bucher N. L. DNA synthesis in primary cultures of adult rat hepatocytes in a defined medium: effects of epidermal growth factor, insulin, glucagon, and cyclic-AMP. J Cell Physiol. 1981 Sep;108(3):353–363. doi: 10.1002/jcp.1041080309. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Gimpel J. A., Deleeuw G., Tischler M. E., Tager J. M., Williamson J. R. Interrelationships between gluconeogenesis and ureogenesis in isolated hepatocytes. J Biol Chem. 1978 Apr 10;253(7):2308–2320. [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- Moreno F., Pastor-Anglada M., Hollenberg M. D., Soley M. Effects of epidermal growth factor (urogastrone) on gluconeogenesis, glucose oxidation, and glycogen synthesis in isolated rat hepatocytes. Biochem Cell Biol. 1989 Oct;67(10):724–729. doi: 10.1139/o89-108. [DOI] [PubMed] [Google Scholar]
- Moule S. K., McGivan J. F. Epidermal-growth-factor stimulation of gluconeogenesis in isolated rat hepatocytes involves the inactivation of pyruvate kinase. Biochem J. 1988 Oct 1;255(1):361–364. [PMC free article] [PubMed] [Google Scholar]
- Noguchi S., Ohba Y., Oka T. Influence of epidermal growth factor on liver regeneration after partial hepatectomy in mice. J Endocrinol. 1991 Mar;128(3):425–431. doi: 10.1677/joe.0.1280425. [DOI] [PubMed] [Google Scholar]
- O'Connor-McCourt M., Soley M., Hayden L. J., Hollenberg M. D. Receptors for epidermal growth factor (urogastrone) and insulin in primary cultures of rat hepatocytes maintained in serum-free medium. Biochem Cell Biol. 1986 Aug;64(8):803–810. doi: 10.1139/o86-108. [DOI] [PubMed] [Google Scholar]
- Pandiella A., Beguinot L., Vicentini L. M., Meldolesi J. Transmembrane signalling at the epidermal growth factor receptor. Trends Pharmacol Sci. 1989 Oct;10(10):411–414. doi: 10.1016/0165-6147(89)90190-9. [DOI] [PubMed] [Google Scholar]
- Pryor H. J., Smyth J. E., Quinlan P. T., Halestrap A. P. Evidence that the flux control coefficient of the respiratory chain is high during gluconeogenesis from lactate in hepatocytes from starved rats. Implications for the hormonal control of gluconeogenesis and action of hypoglycaemic agents. Biochem J. 1987 Oct 15;247(2):449–457. doi: 10.1042/bj2470449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan P. T., Halestrap A. P. The mechanism of the hormonal activation of respiration in isolated hepatocytes and its importance in the regulation of gluconeogenesis. Biochem J. 1986 Jun 15;236(3):789–800. doi: 10.1042/bj2360789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed S. M., Patel T. B. Regulation of hepatic energy metabolism by epidermal growth factor. Eur J Biochem. 1991 May 8;197(3):805–813. doi: 10.1111/j.1432-1033.1991.tb15975.x. [DOI] [PubMed] [Google Scholar]
- Sistare F. D., Haynes R. C., Jr The interaction between the cytosolic pyridine nucleotide redox potential and gluconeogenesis from lactate/pyruvate in isolated rat hepatocytes. Implications for investigations of hormone action. J Biol Chem. 1985 Oct 15;260(23):12748–12753. [PubMed] [Google Scholar]
- Soler C., Poveda B., Pastor-Anglada M., Soley M. Effect of epidermal growth factor (EGF) on gluconeogenesis in isolated rat hepatocytes. Dependency on the red-ox state of the substrate. Biochim Biophys Acta. 1991 Jan 31;1091(2):193–196. doi: 10.1016/0167-4889(91)90061-2. [DOI] [PubMed] [Google Scholar]
- Soler C., Poveda B., Soley M., Pastor-Anglada M. Effect of epidermal growth factor on ornithine decarboxylase activity and urea synthesis in isolated rat hepatocytes. Cell Mol Biol. 1992 May;38(3):281–288. [PubMed] [Google Scholar]
- Soley M., Hollenberg M. D. Epidermal growth factor (urogastrone)-stimulated gluconeogenesis in isolated mouse hepatocytes. Arch Biochem Biophys. 1987 May 15;255(1):136–146. doi: 10.1016/0003-9861(87)90303-1. [DOI] [PubMed] [Google Scholar]
- St Hilaire R. J., Hradek G. T., Jones A. L. Hepatic sequestration and biliary secretion of epidermal growth factor: evidence for a high-capacity uptake system. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3797–3801. doi: 10.1073/pnas.80.12.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano T., Shiota M., Tanaka T., Miyamae Y., Shimada M., Oshino N. Intracellular redox state and stimulation of gluconeogenesis by glucagon and norepinephrine in the perfused rat liver. J Biochem. 1980 Jan;87(1):153–166. doi: 10.1093/oxfordjournals.jbchem.a132721. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K., Nakamura T., Ichihara A. Reciprocal effects of epidermal growth factor on key lipogenic enzymes in primary cultures of adult rat hepatocytes. Induction of glucose-6-phosphate dehydrogenase and suppression of malic enzyme and lipogenesis. J Biol Chem. 1983 Oct 25;258(20):12355–12360. [PubMed] [Google Scholar]
- Zahlten R. N., Stratman F. W., Lardy H. A. Regulation of glucose synthesis in hormone-sensitive isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3213–3218. doi: 10.1073/pnas.70.11.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]


