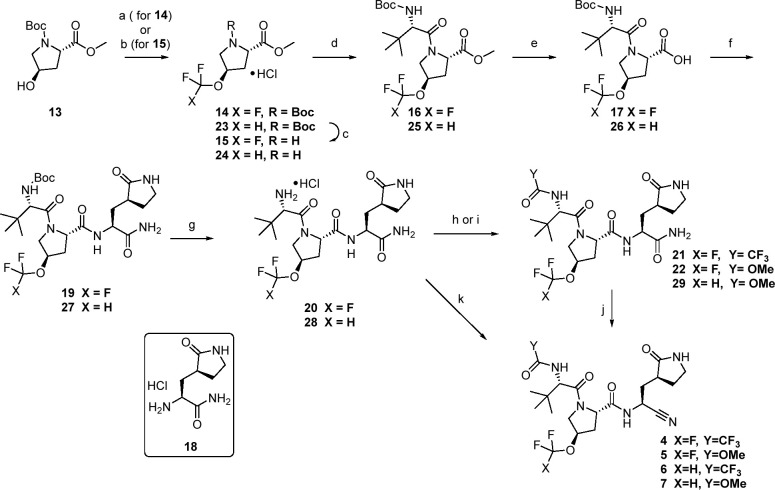
Scheme 1. Synthesis of Fluorinated Ether Analogs 4–7.
Reagents and conditions: (a) AgOTf, Selectfluor, KF, TMSCF3, 2-F-Py, EtOAc 29 °C, 21%; (b) 2-(fluorosulfonyl)difluoroacetic acid, CuI, CH3CN, 50 °C, 63%; (c) representative conditions: 4 N HCl in dioxane, 93%; (d) N-Boc-l-tert-leucine, HATU, i-Pr2NEt, DMF, 73–84%; (e) LiOH·H2O, CH3OH or THF, H2O, 0–25 °C, 87–92%; (f) 18, HATU, i-Pr2NEt, DMF, 0–25 °C 59–82%; (g) HCl in ethyl acetate, 94–100%; (h) TFAA, Et3N, DCM, 0–20 °C, 40%; (i) methylchloroformate, Et3N, DCM; (j) Burgess reagent, DCM, 21–26%; (k) TFAA, NMM, i-PrOAc, 0 °C, 73%.