Figure 1. Pre-F vaccination elicits a greater magnitude of pre-F-exclusive and highly neutralizing antibodies than post-F vaccination.
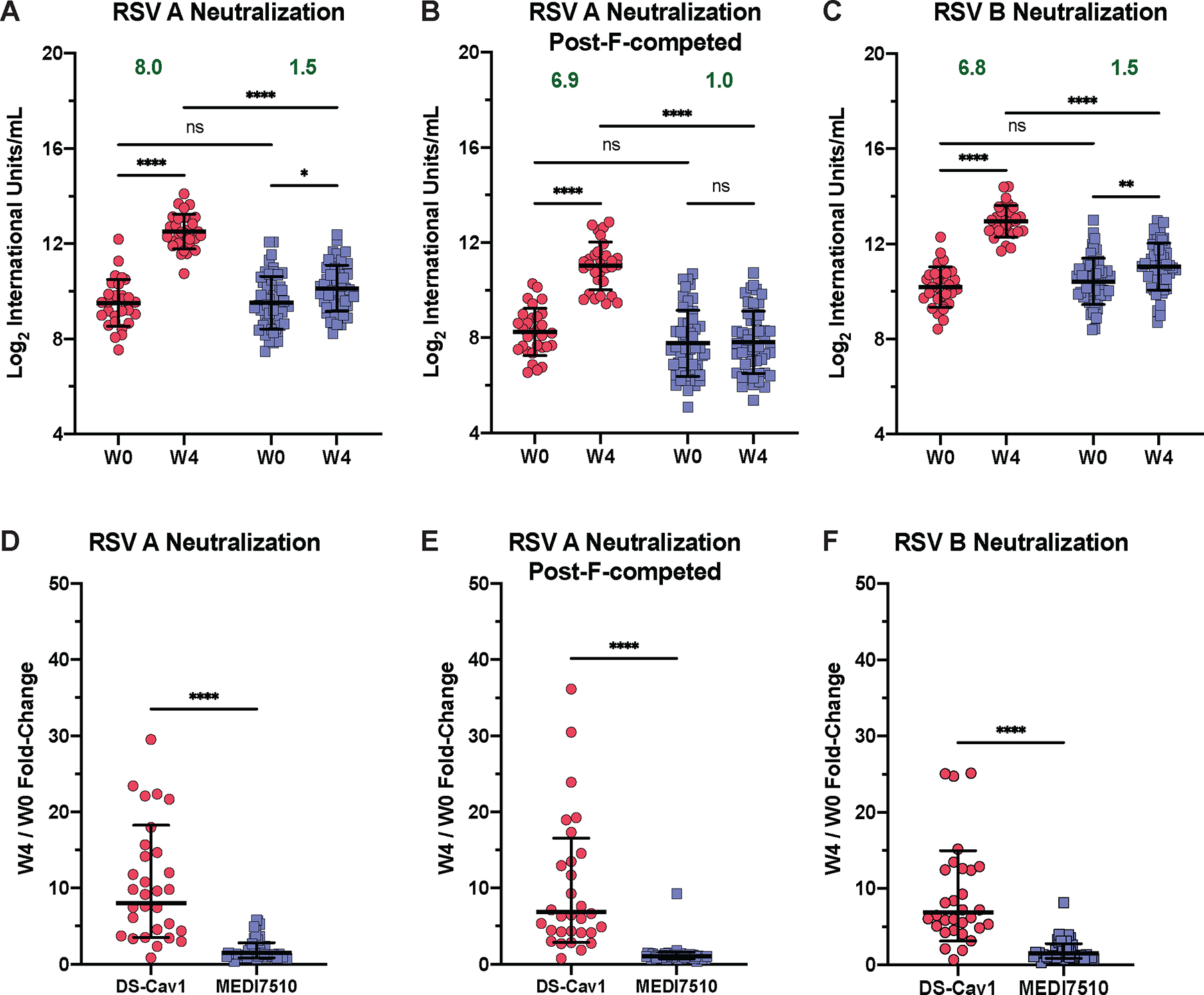
(A to C) Conformation-specific neutralization capacity of serum obtained at week 0 (W0) and week 4 (W4) after vaccination with DS-Cav1 (pre-F, N = 30) or MEDI7510 (post-F, N = 52) was measured for (A) RSV subtype A2 without post-F competition or (B) with post-F competition, and (C) subtype B. In (B), excess post-F was used to adsorb post-F-exclusive and dual-binding antibodies to assess the neutralization capacity of pre-F exclusive antibodies in serum. (D to F) Fold-change in neutralization capacity at W4 after vaccination with either DS-Cav1 or MEDI7510 was calculated for RSV subtype A2 (D) without or (E) with post-F competition, and (F) subtype B. Bars represent mean ± standard deviation (SD) of log2 data. Dotted lines indicate the limit of detection (LOD) for each assay. For all graphs, asterisks denote the P-value, with ****P < 0.0001, ***P = 0.0001 to 0.001, **P = 0.001 to 0.01, *P = 0.01 to 0.05, and ns = not significant as determined using Brown-Forsythe and Welch ANOVA tests (A to C), Kruskal-Wallis tests (D and E), or Mann-Whitney tests (F). Red circles denote participants who received DS-Cav1, blue squares denote participants who received MEDI7510. Geometric mean W4/W0 fold-change is shown in green text.
