Abstract
Background
Protein biomarkers have been broadly investigated in cerebrospinal fluid and blood for the detection of neurodegenerative diseases, yet a clinically useful diagnostic test to detect early, pre-symptomatic Alzheimer’s disease (AD) remains elusive. We conducted this study to quantify Aβ40, Aβ42, total Tau (t-Tau), hyperphosphorylated Tau (ptau181), glial fibrillary acidic protein (GFAP) and neurofilament light chain (NfL) in eye fluids relative to blood.
Methods
In this cross-sectional study we collected vitreous humor, aqueous humor, tear fluid and plasma in patients undergoing surgery for eye disease. All six biomarkers were quantitatively measured by digital immunoassay. Spearman and Bland–Altman correlation analyses were performed to assess the agreement of levels between ocular fluids and plasma.
Results
Seventy-nine adults underwent pars-plana vitrectomy in at least one eye. Of the 79, there were 77 vitreous, 67 blood, 56 tear fluid, and 51 aqueous samples. All six biomarkers were quantified in each bio-sample, except GFAP and NfL in tear fluid due to low sample volume. All six biomarkers were elevated in vitreous humor compared to plasma samples. T-Tau, ptau181, GFAP and NfL were higher in aqueous than in plasma, and t-Tau and ptau181 concentrations were higher in tear fluid than in plasma. Significant correlations were found between Aβ40 in plasma and tears (r = 0.5; p = 0.019), t-Tau in plasma and vitreous (r = 0.4; p = 0.004), NfL in plasma and vitreous (r = 0.3; p = 0.006) and plasma and aqueous (r = 0.5; p = 0.004). No significant associations were found for Aβ42, ptau181 and GFAP among ocular fluids relative to plasma. Bland–Altman analysis showed aqueous humor had the closest agreement to plasma across all biomarkers. Biomarker levels in ocular fluids revealed statistically significant associations between vitreous and aqueous for t-Tau (r = 0.5; p = 0.001), GFAP (r = 0.6; p < 0.001) and NfL (r = 0.7; p < 0.001).
Conclusion
AD biomarkers are detectable in greater quantities in eye fluids than in plasma and show correlations with levels in plasma. Future studies are needed to assess the utility of ocular fluid biomarkers as diagnostic and prognostic markers for AD, especially in those at risk with eye disease.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-024-01556-y.
Keywords: Alzheimer’s disease, Aqueous humor, Tears, Vitreous humor, Amyloid beta, Tau, Glial fibrillary acidic protein, Neurofilament light chain
Background
Alzheimer’s Disease (AD) is the most common cause of dementia in the elderly. With an expected prevalence of 13.8 million individuals in the United States (US) by 2060 [1] and 152 million worldwide by 2050 [2], AD is a neurodegenerative disease characterized by the accumulation of neuritic amyloid plaques and hyperphosphorylated tau. The current diagnostic protocol includes functional assessment and neuropsychological testing, if warranted. Cerebrospinal fluid (CSF) analysis and central nervous system (CNS) imaging may be used to aid in the diagnosis. However, the expensive and potentially invasive nature of this testing precludes its broad application and use as a screening tool for early diagnosis. Given that newly approved treatments may slow progression of AD, it will be vital to develop diagnostic methods that are widely available and can identify the disease in its earliest stages.
In recent years, blood-based biomarkers (BBB) from plasma and serum are showing promise for the diagnosis of AD [3, 4]. BBB can potentially be used by both specialized and primary care clinics to test the heterogenous older population at risk for AD and may represent a valuable screening tool for clinical trials to identify asymptomatic AD patients. However, the complex composition of blood makes it difficult to develop both sensitive and specific markers for preclinical AD diagnosis.
Those with eye disease are at increased risk for developing AD. Former studies have shown that patients with eye disease, such as diabetic retinopathy, age-related macular degeneration, and glaucoma, confer a higher risk of AD development [5, 6]. The eye and brain share a common anatomic and developmental origin [7], and recent research has focused on ocular fluids as a potential reservoir for neurodegenerative biomarkers for early AD diagnosis. In prior studies, our group has demonstrated measurable concentrations of amyloid β (Aβ)40, Aβ42, hyperphosphorylated tau 181 (ptau181), total tau (t-Tau), and neurofilament light chain (NfL) in the vitreous humor [8–10].
While vitreous fluid can be collected in either the clinic or operating room, other ocular fluids such as aqueous humor or tear fluid are more easily accessible. Other groups have successfully measured concentrations of amyloid, tau, NfL miRNAs, translation initiation factors and lipoproteins in both aqueous humor [11–14] and tears [15–18]. The purpose of this study is to explore the relative concentrations of Aβ40, Aβ42, ptau181, t-Tau, glial fibrillary acidic protein (GFAP) and NfL in three different ocular fluids: vitreous humor, aqueous humor, and tear fluid, and to reference them to plasma levels in the same cohort of patients.
Methods
This prospective, cross-sectional cohort study was conducted at Boston University Medical Center (BUMC). Approval and oversight for the study protocol were provided by the BUMC Institutional Review Board (study reference number H-37370, principal investigator MLS) and the study was carried out in accordance with the ethical standards of the Committee on Human Experimentation of our institution and the Declaration of Helsinki.
Inclusion criteria included participants aged 18 years or older with a primary language of English or Spanish requiring pars plana vitrectomy (PPV) for ophthalmic disease. Surgical indications for PPV included rhegmatogenous retinal detachment (RRD), macular hole (MH), epiretinal membrane (ERM), and complications of diabetic retinopathy (DR) such as vitreous hemorrhage (VH) and tractional retinal detachment (TRD). Written informed consent was obtained from all patients who participated in the study, and no individuals were excluded due to existing ocular or medical comorbidities.
Demographic and clinical data were obtained through the completion of a patient questionnaire as well as by review of patients’ electronic medical records in a standardized manner. Demographic data included age, sex, self-declared ethnicity, and the highest educational level completed, along with athletic and military history. Clinical information was collected on study participants, including medical and smoking history, history of head and/or neck injuries, family history of cognitive dysfunction, and subjective cognitive complaints. Furthermore, baseline color vision, ocular history, and family history of ocular disease were obtained.
Biospecimen collection
Vitreous samples were collected at the start of each vitrectomy procedure with 0.5–1.0 mL of undiluted vitreous fluid aspirated via the vitrectomy probe into an attached sterile 3-mL syringe. Infusion of balanced salt solution into the vitreous cavity was immediately undertaken in order to re-pressurize the eye. Aqueous samples were collected through the limbus prior to initiating vitrectomy using a 1 cc syringe and hypodermic needle (30 gauge) to aspirate 100–150 µl of undiluted aqueous. Balanced salt solution was used to reform the anterior chamber. The syringes containing both eye fluid specimens were capped using sterile technique and directly handed to a research assistant who labeled them with a predetermined non-identifiable study number and placed the samples on ice. In the Molecular Genetics Core Laboratory (MGCL) at Boston University Medical Center, vitreous and aqueous fluid were centrifuged for 15 min at 12,000 rpm to separate the cellular contents, divided into 100 μl aliquots, and frozen at – 80 °C. Aside from the collection of the vitreous and aqueous samples, each study participant’s vitrectomy was completed according to the clinical standard of care for that patient’s ocular condition.
Tear fluid and blood samples were collected on separate clinical visits from all study participants within 1–2 months of collection of vitreous and aqueous samples. Tear fluid was collected from both eyes via Schirmer’s tear strips (Eye Care and Cure Corp., AZ) without topical anesthesia. The strips were placed between the palpebral and bulbar conjunctiva in the inferior sac for 2 min [19] with the eyes closed. We used multiple Schirmer strips on both eyes of each participant within the 2-min window. To maximize tear fluid collection, we used multiple rounds of collection with Schirmer’s strips, up to 45 min for as long as the participant was willing to tolerate it. The soaked Schirmer strips were placed in a punctured 0.5 ml tube, and these 0.5 ml tubes were further placed inside 1.5 ml Eppendorf tubes. Then the soaked strips were centrifuged to separate the fluid from the strip into the 1.5 ml tubes through the pores of 0.5 ml tubes and aliquoted with micropipettes. Tear fluid samples were stored at – 80 °C until time of analysis. For the blood samples, eighteen milliliters of whole blood was drawn from each patient into EDTA-treated purple top tubes. The MGCL processed the de-identified blood samples into their component serum, plasma, and buffy coat.
Immunoassay measurements
Vitreous, aqueous, tear fluid, and plasma samples were tested for Aβ40, Aβ42, ptau181, t-Tau, GFAP, and NfL. Briefly, Aβ42, Aβ40, and t-Tau relative concentrations were measured using Neurology 3-Plex A Assay (#101,995, Quanterix, MA) with a 4- fold dilution on HD-X analyzer (Quanterix). ptau181 levels were measured using ptau181 V2 Advantage kit (#103,714, Quanterix, MA) with a 4- fold dilution. Concentrations of GFAP and NfL were measured using the combined Neurology 2-Plex B assay (#103,520, Quanterix, MA) with a fourfold dilution for vitreous and plasma and an eightfold dilution for aqueous samples. All samples were processed per manufacturer’s instructions in the immunoassay kits.
There were insufficient tear fluid volumes to allow for testing of all biomarkers (Table 1). Based on results from our prior studies, we prioritized the biomarker assays for tears to focus on pTau181, total tau, Aβ40 and Aβ42.
Table 1.
Biospecimen volume collections
| Biospecimen | Volume (μL) | ||
|---|---|---|---|
| Mean | Median | Range | |
| Blood | |||
| Plasma | 2,670 | 3,150 | 0–8,100 |
| Ocular fluids | |||
| Vitreous Humor | 1,111 | 1,085 | 250–2,150 |
| Aqueous Humor | 109 | 120 | 0–320 |
| Tear Fluid | 79 | 75 | 0–180 |
Data is reported as volume values in microliter (μL)
Statistical analysis
The level and spread of each biomarker within each biofluid were summarized by reporting means, medians, and interquartile ranges (IQR). Because of skewness in the distribution of biomarkers, we used nonparametric Spearman’s rank rho correlation coefficient to assess the relationship among the relative concentration of each neurodegenerative biomarker in different ocular fluids. The correlations may be interpreted as weak, moderate, good, or excellent based on correlation coefficient values of less than 0.50, 0.50 to 0.75, 0.76 to 0.90, or 0.91 to 1.00, respectively, based on previously described guidelines [20]. We used Bland–Altman analysis to quantify the agreement of biomarker levels between plasma and the three ocular fluids. Bias, defined as the average pair-wise difference in plasma biomarker levels and one of the three other biomarker levels, and 95% limits of agreement (LOA) are estimated using a regression approach [21]; this accounts for the complex relationship between bias and magnitude of plasma biomarker levels. All P values ≤ 0.05 were considered significant for these exploratory analyses, and those between 0.05 and 0.1 were considered trends.
Results
We enrolled 79 eyes of 79 adults who underwent pars plana vitrectomy. Of the 79 patients who underwent sample collection, we ultimately collected 77 vitreous humor samples, 67 blood samples, 56 tear fluid samples, and 51 aqueous humor samples. Missing samples were due to various difficulties, including challenges with specimen collection, patients refusing blood draws, and inadequate sample volume (common with tear fluid and aqueous fluid). Study population characteristics are displayed in Table 2. Participants had a mean age of 57.6 ± 12.2 years at the time of consent into the study and 40.5% were female. The ethnic breakdown of our study population is a close representation of the patient population typically seen at the eye clinic at BUMC with 53.2% reporting not Hispanic/Latino in ethnicity.
Table 2.
Demographic participants’ characteristics
| Parameter | N (%) or Mean ± SD |
|---|---|
| Participants (Eyes N = 79) | 79 |
| Age (years) | 57.6 ± 12.2 |
| Female Gender | 32 (40.5) |
| Ethnicity | |
| Hispanic/Latino | 36 (45.6) |
| Not Hispanic/Latino | 42 (53.2) |
| Not reported/Missing | 1 (1.3) |
Data is reported as number with (%) or mean with standard deviation (SD), as appropriate
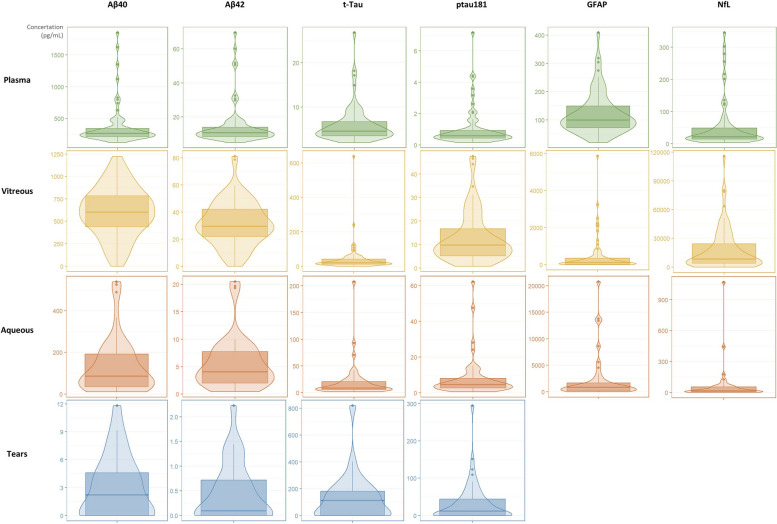
All six biomarkers (Aβ40, Aβ42, ptau181, t-Tau, GFAP and NfL) were detectable and quantified in samples of plasma, vitreous, and aqueous (Supplementary Table 1). All biomarkers with the exception of GFAP and NfL were detected in tear fluid samples (due to low sample volume) (Supplementary Table 1). Measured concentrations of all six biomarkers were higher in vitreous humor than plasma samples, with amyloid levels approximately twofold higher in vitreous than in plasma, and larger differences for tau, GFAP and NfL. Measured levels of t-Tau, ptau181, GFAP and NfL were higher in aqueous than in plasma, and t-Tau and ptau181 concentrations were higher in tear fluid than in plasma (Fig. 1).
Fig. 1.
Violin box plots for the distribution of the relative concentrations (pg/mL) of each biomarker among the different biofluids. Bottom and top lines of the boxes correspond to first and third quartiles respectively, and the middle lines refers to the mean value. The density plots represent an estimation that shows the distribution shape of the data and its bottom and top points correspond to the zero and fourth quartiles respectively. Aβ: amyloid beta; t-Tau: total tau; ptau181: hyperphosphorylated tau 181; GFAP: Glial fibrillary acidic protein; NfL: neurofilament light chain
Spearman’s rank correlations coefficients analysis revealed significant correlations between Aβ40 levels in plasma and tears (r = 0.5; p = 0.019) and between t-Tau levels in plasma and vitreous (r = 0.4; p = 0.004) (Table 3). Significant correlations were also detected for NfL levels between plasma and vitreous (r = 0.3; p = 0.006) and plasma and aqueous (r = 0.5; p = 0.004) (Table 3). No significant associations were found for Aβ42, ptau181 and GFAP levels among any of the ocular biosamples in relation to the plasma (Table 3). Further analysis comparing biomarker levels in ocular fluids revealed statistically significant associations for t-Tau (r = 0.5; p = 0.001), GFAP (r = 0.6; p < 0.001) and NfL (r = 0.7; p < 0.001) levels in vitreous and aqueous (Table 4).
Table 3.
Correlation analysis of the biomarkers’ relative concentrations between eye fluids and plasma
| Aβ40 | Aβ42 | t-Tau | ptau181 | GFAP | NfL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison | R-value | P-value | R-value | P-value | R-value | P-value | R-value | P-value | R-value | P-value | R-value | P-value |
| Vitreous—Plasma | 0.10 | 0.42 | 0.14 | 0.27 | 0.36 | 0.004* | 0.17 | 0.18 | 0.06 | 0.6 | 0.33 | 0.006* |
| Aqueous—Plasma | 0.26 | 0.12 | 0.26 | 0.12 | -0.09 | 0.60 | 0.17 | 0.27 | 0.24 | 0.17 | 0.49 | 0.004* |
| Tears—Plasma | 0.50 | 0.02* | 0.34 | 0.13 | 0.08 | 0.74 | 0.11 | 0.49 | NA | NA | NA | NA |
Spearman’s rank correlations coefficients rho (R) and P values for eye fluids against plasma. ‘*’ indicates statistical significance with P value lower or equal to 0.05. Aβ: amyloid beta; t-Tau: total tau; ptau181: hyperphosphorylated tau 181; GFAP: Glial fibrillary acidic protein; NfL: neurofilament light chain
Table 4.
Correlation analysis of the biomarkers’ relative concentrations across inter-eye chamber fluids
| Aβ40 | Aβ42 | t-Tau | ptau181 | GFAP | NfL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison | R-value | P-value | R-value | P-value | R-value | P-value | R-value | P-value | R-value | P-value | R-value | P-value |
| Vitreous—Aqueous | 0.11 | 0.49 | 0.10 | 0.52 | 0.47 | 0.001* | 0.28 | 0.05 | 0.60 | < 0.001* | 0.68 | < 0.001* |
| Vitreous—Tears | -0.19 | 0.34 | -0.16 | 0.45 | 0.16 | 0.43 | -0.19 | 0.17 | NA | NA | NA | NA |
| Tears—Aqueous | 0.31 | 0.37 | 0.16 | 0.64 | 0.23 | 0.49 | 0.02 | 0.91 | NA | NA | NA | NA |
Spearman’s rank correlations coefficients rho (R) and P values for inter-eye chamber fluids. ‘*’ indicates statistical significance with P value lower or equal to 0.05. Aβ amyloid beta, t-Tau total tau, ptau181 hyperphosphorylated tau 181, GFAP Glial fibrillary acidic protein, NfL neurofilament light chain
Bland–Altman analysis was used to assess agreement between pairwise differences in participants’ plasma biomarker and vitreous humor, aqueous humor, and tear fluid respectively (Table 5). Low bias, meaning biomarker closer to plasma biomarker and small 95% limits of agreement (95% LOA), indicates better precision. Overall, we saw better agreement (low bias or low mean difference in pairwise levels of biomarker) between aqueous humor and plasma across all biomarkers. Tear fluid showed the largest differences from plasma for Aβ40, t-Tau, and ptau181 and vitreous humor showed the largest differences for Aβ42 (Table 5).
Table 5.
Agreement analysis of the biomarkers’ relative concentrations between eye fluids and plasma
| Aβ40 | Aβ42 | t-Tau | ptau181 | GFAP | NfL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison | Bias | 95% LOA | Bias | 95% LOA | Bias | 95% LOA | Bias | 95% LOA | Bias | 95% LOA | Bias | 95% LOA |
| Aqueous—Plasma | 229.2 | 504.7 | 8.5 | 18.0 | -14.2 | 142.0 | -7.2 | 47.4 | -1796.4 | 13,296.8 | -16.5 | 762.0 |
| Vitreous—Plasma | -258.6 | 1079.7 | -18.6 | 63.6 | -33.3 | 319.7 | -11.7 | 42.0 | -17,863.1 | 87,989.4 | -364.9 | 3517.7 |
| Tears—Plasma | 311.0 | 11.2 | 12.2 | 1.9 | -89.9 | 383.9 | -31.2 | 200.7 | NA | NA | NA | NA |
Bland–Altman agreement analysis, showing bias (mean difference) and 95% limits of agreement (LOA) between pairwise differences in levels of eye-fluid biomarker and magnitude of plasma biomarker. Aβ amyloid beta, t-Tau total tau, ptau181 hyperphosphorylated tau 181, GFAP Glial fibrillary acidic protein, NfL neurofilament light chain
Discussion
In this study, we detected and quantified Aβ40, Aβ42, t-Tau, ptau181, GFAP, and NfL in the vitreous humor, aqueous humor, and plasma. Additionally, we detected all biomarkers that could be tested in tear fluid (Aβ40, Aβ42, t-Tau, and ptau181) except for GFAP and NfL, due to low sample volume. Aqueous humor showed the best agreement with plasma levels across all biomarkers. As far as we are aware, this study is the first to measure and correlate concentrations of neurodegenerative protein biomarkers from three different ocular fluid sources and associate their levels with the blood in a single cohort of participants.
Based on the close embryological and functional relationship of the eye and brain, recent research has focused on the correlation of ocular biomarkers and neurodegenerative diseases. Ocular imaging studies using high resolution non-contact optical coherence tomography (OCT) devices have identified structural changes including thinning of the retinal nerve fiber layer (RNFL), reduced retinal volume, and thinning of the choroidal layer [9, 22–32] in association with AD. Additionally, studies using optical coherence tomography angiography (OCTA) have demonstrated measurable alterations in the retinal vascular plexuses including enlargement of the foveal avascular zone [33–37] in patients with preclinical AD. While imaging-based eye biomarkers are promising, their clinical utility may be limited by the presence of common comorbidities such as diabetes, hypertension, and glaucoma, which can contribute to retinal vascular and anatomical alterations [10, 38–40].
In addition to retinal imaging studies, research has focused on the detection of AD-associated proteins in the aqueous and vitreous humor [41–44]. We have previously reported decreased vitreous levels of Aβ40, Aβ42, and tTau in patients with lower mini-mental state examination (MMSE) scores [45]. Goldstein et al. identified Aβ1-40 concentrations in aqueous humor comparable to CSF levels in AD patients [46]. Janciauskiene et al. detected increased levels of amyloid and neuroinflammatory cytokines in aqueous humor of patients with age-related eye diseases who underwent cataract surgery [13]. Bai et al. measured NfL, Aβ40, Aβ42, GFAP, and ptau181 in aqueous samples and found that NfL was negatively correlated with lower MMSE scores and lower vessel density on the superficial capillary plexus on OCTA [11]. Disease biomarkers can also be found in ocular tissues, as Aβ deposits have been reported in the lens and retina in both animal models [47–51] and human studies [46, 52–55].
While in-office needle aspiration of both aqueous and vitreous fluid is possible, tear fluid offers the advantage of non-invasive collection [56]. Previous studies have demonstrated increased levels of tear fluid amyloid, tau, miRNAs, translation initiation factors and lipoproteins in AD patients compared to controls [15–18]. While Schirmer strip tear fluid sampling in our study may have been limited by evaporation, other methods such as capillary tube collection may offer better yields by limiting the time tear fluid is exposed to air [57]. Certainly, the accessibility and cost effectiveness of tear fluid collection make it an attractive avenue for future biomarker research.
Our study has limitations in that all participants included had retinal disease requiring treatment with vitrectomy surgery. Given that patients with eye disease represent an at-risk population for AD, it will be important for future studies looking at ocular fluid biomarker as potential diagnostic tools to assess their correlation with ophthalmic conditions. Although some correlations between the ocular fluids and plasma showed statistical significance, some correlation strengths were weak. Nevertheless, the effect sizes that we observe in this study are clinically significant and similar to what we observe in similar cognitive studies [58, 59]. It is also important to recognize that no subject in our study had a formal dementia diagnosis, so this study does not allow us to speculate on any correlation between ocular fluid biomarker levels and clinical diagnosis of dementia. Future studies should examine associations between ocular fluid biomarkers and cognitive impairment.
Additionally, our study was limited by difficulty with tear fluid collection. Due to small tear volumes, we were not able to measure GFAP and NfL levels, and while the relative concentrations of t-Tau and ptau181 were higher in tears than in any other compartment, it is possible that tear evaporation may have altered protein concentration resulting in false measurements. Finally, our sample size of 79 eyes is limited, and a larger sample may have provided more significant associations.
Conclusion
In summary, levels of the AD biomarkers Aβ40, Aβ42, t-Tau, ptau181, GFAP, and NfL were detectable in greater concentrations in eye fluids than in plasma. Aβ40, t-Tau, and NfL showed significant correlations with plasma levels, and levels in the aqueous humor showed the greatest agreement with plasma levels across all biomarkers. Future studies on ocular fluid biomarkers should focus on the role of eye diseases and include participants with cognitive impairment and dementia in order to assess their potential utility as diagnostic and prognostic markers for AD.
Supplementary Information
Authors’ contributions
KS, SN, TDS, MLS contributed equally to this work. KS contributed to the interpretation of the data, created the tables, wrote and revised the manuscript. SN performed the vitrectomy surgery, collected the vitreous and aqueous humor specimens during surgery, and contributed to the manuscript revisions and final approval. FTZ performed the statistical analysis, created the violin box plots with the biomarker distributions in each biospecimen, and wrote the Bland–Altman analysis results in the results section. NA and EES conducted the immunoassays for all the biomarkers. SA was involved with patient enrolment and consent, tear fluid sample collection, data entry and dataset management, project coordination, and manuscript revision. XC and NHS performed the vitrectomy surgery, collected the vitreous and aqueous humor specimens during surgery, and contributed to the manuscript revisions. MLA contributed to the manuscript revisions. WX aided in the immunoassays of proteins in all biospecimens (vitreous, aqueous, tears, blood), and contributed to the interpretation of the data. YT contributed to the interpretation of the data, revised the statistical analysis and provided manuscript revisions. TDS is the corresponding co-author, contributed to the study conception and design, aided in the immunoassays of proteins in all biospecimens (vitreous, aqueous, tears, blood), contributed to the interpretation of the data, and critically revised and approved the manuscript. MLS is the principal investigator and the corresponding co-author. MLS was responsible for the study conception and design, performed the vitrectomy surgery, collected the vitreous and aqueous humor samples during surgery, interpreted the data, and critically revised the manuscript. The author(s) read and approved the final manuscript.
Funding
This research was funded by NIH/NIA/ERP: 1R03AG063255-01, PI Manju Subramanian; P30AG072978, the United States Department of Veterans Affairs, Veterans Health Administration, BLRD Merit Award (I01BX005933).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Data availability
Data is provided within the manuscript. Moreover, the datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participate
The Boston University Medical Center/Boston University Medical Campus Institutional Review Board reviewed and approved conduct of this research study (BMC IRB# H-37370). The study was conducted in accordance with Declaration of Helsinki. The study participants provided their written consent prior to participating in the study activities.
Consent for publication
Not applicable here.
Competing interests
KS, SN, FTZ, NA, EES, XC, NHS, YT, TDS, MLS declare that they have no competing interests. MLA received a single time honorarium from the Michael J Fox Foundation for services unrelated to this study. MLA also receives royalties from Oxford University Press Inc.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Konstantina Sampani, Steven Ness, Thor D. Stein and Manju L. Subramanian contributed equally to this work.
Contributor Information
Thor D. Stein, Email: tdstein@bu.edu
Manju L. Subramanian, Email: manju.subramanian@bmc.org
References
- 1.Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19(4):1598–695. 10.1002/alz.13016 [DOI] [PubMed] [Google Scholar]
- 2.Collaborators, G.B.D.D.F. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–25. 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson O, et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement. 2022;18(12):2669–86. 10.1002/alz.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teunissen CE, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66–77. 10.1016/S1474-4422(21)00361-6 [DOI] [PubMed] [Google Scholar]
- 5.Lee CS, et al. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement. 2019;15(1):34–41. 10.1016/j.jalz.2018.06.2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang PH, et al. Ophthalmic conditions associated with dementia risk: The Cardiovascular Health Study. Alzheimers Dement. 2021;17(9):1442–51. 10.1002/alz.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory RL. Origin of eyes and brains. Nature. 1967;213(5074):369–72. 10.1038/213369a0 [DOI] [PubMed] [Google Scholar]
- 8.Subramanian ML, et al. Neurofilament light chain in the vitreous humor of the eye. Alzheimers Res Ther. 2020;12(1):111. 10.1186/s13195-020-00677-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheikh Z, et al. The association of vitreous biomarker levels and optical coherence tomography markers to cognitive status. Invest Ophthalmol Vis Sci. 2023;64(8):5276. [Google Scholar]
- 10.Vig V, et al. Vitreous humor biomarkers reflect pathological changes in the brain for Alzheimer’s disease and chronic traumatic encephalopathy. J Alzheimers Dis. 2023;93(3):1181–93. 10.3233/JAD-230167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai J, et al. Association of cognitive function with neurofilament light chain in the aqueous humor of human eye. Front Aging Neurosci. 2022;14:1027705. 10.3389/fnagi.2022.1027705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwak DE, et al. Alterations of aqueous humor Abeta levels in Abeta-infused and transgenic mouse models of Alzheimer disease. PLoS ONE. 2020;15(1):e0227618. 10.1371/journal.pone.0227618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janciauskiene S, et al. Detection of Alzheimer peptides and chemokines in the aqueous humor. Eur J Ophthalmol. 2011;21(1):104–11. 10.5301/EJO.2010.2108 [DOI] [PubMed] [Google Scholar]
- 14.Inoue T, Kawaji T, Tanihara H. Elevated levels of multiple biomarkers of Alzheimer’s disease in the aqueous humor of eyes with open-angle glaucoma. Invest Ophthalmol Vis Sci. 2013;54(8):5353–8. 10.1167/iovs.13-12245 [DOI] [PubMed] [Google Scholar]
- 15.Kallo G, et al. Changes in the chemical barrier composition of tears in Alzheimer’s disease reveal potential tear diagnostic biomarkers. PLoS ONE. 2016;11(6):e0158000. 10.1371/journal.pone.0158000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny A, et al. Proteins and microRNAs are differentially expressed in tear fluid from patients with Alzheimer’s disease. Sci Rep. 2019;9(1):15437. 10.1038/s41598-019-51837-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Prete S, Marasco D, Sabetta R, Del Prete A, Marino FZ, Franco R, Troisi S, Troisi M, Cennamo G. Tear Liquid for Predictive Diagnosis of Alzheimer’s Disease. Reports. 2021;4(3):26. 10.3390/reports4030026. 10.3390/reports4030026 [DOI] [Google Scholar]
- 18.Gijs M, et al. Association of tear fluid amyloid and tau levels with disease severity and neurodegeneration. Sci Rep. 2021;11(1):22675. 10.1038/s41598-021-01993-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karampatakis V, et al. Comparison between normal values of 2- and 5-minute Schirmer test without anesthesia. Cornea. 2010;29(5):497–501. 10.1097/ICO.0b013e3181c2964c [DOI] [PubMed] [Google Scholar]
- 20.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–63. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60. 10.1177/096228029900800204 [DOI] [PubMed] [Google Scholar]
- 22.Oktem EO, et al. The relationship between the degree of cognitive impairment and retinal nerve fiber layer thickness. Neurol Sci. 2015;36(7):1141–6. 10.1007/s10072-014-2055-3 [DOI] [PubMed] [Google Scholar]
- 23.Cunha LP, et al. Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimer’s disease. PLoS ONE. 2016;11(4):e0153830. 10.1371/journal.pone.0153830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Martin ES, et al. Macular thickness as a potential biomarker of mild Alzheimer’s disease. Ophthalmology. 2014;121(5):1149-1151 e3. 10.1016/j.ophtha.2013.12.023 [DOI] [PubMed] [Google Scholar]
- 25.den Haan J, et al. Retinal thickness correlates with parietal cortical atrophy in early-onset Alzheimer’s disease and controls. Alzheimers Dement (Amst). 2018;10:49–55. 10.1016/j.dadm.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Martin E, et al. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer’s disease. Acta Ophthalmol. 2016;94(6):e454–9. 10.1111/aos.12977 [DOI] [PubMed] [Google Scholar]
- 27.Cunha JP, et al. OCT in Alzheimer’s disease: thinning of the RNFL and superior hemiretina. Graefes Arch Clin Exp Ophthalmol. 2017;255(9):1827–35. 10.1007/s00417-017-3715-9 [DOI] [PubMed] [Google Scholar]
- 28.Liu S, et al. The association between retinal neuronal layer and brain structure is disrupted in patients with cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2016;54(2):585–95. 10.3233/JAD-160067 [DOI] [PubMed] [Google Scholar]
- 29.Trebbastoni A, et al. Attenuation of choroidal thickness in patients with Alzheimer disease: evidence from an Italian prospective study. Alzheimer Dis Assoc Disord. 2017;31(2):128–34. 10.1097/WAD.0000000000000176 [DOI] [PubMed] [Google Scholar]
- 30.Bayhan HA, et al. Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin Exp Ophthalmol. 2015;43(2):145–51. 10.1111/ceo.12386 [DOI] [PubMed] [Google Scholar]
- 31.Gharbiya M, et al. Choroidal thinning as a new finding in Alzheimer’s disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J Alzheimers Dis. 2014;40(4):907–17. 10.3233/JAD-132039 [DOI] [PubMed] [Google Scholar]
- 32.Cheung CY, et al. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2015;45(1):45–56. 10.3233/JAD-141659 [DOI] [PubMed] [Google Scholar]
- 33.O’Bryhim BE, et al. Association of preclinical Alzheimer disease with optical coherence tomographic angiography findings. JAMA Ophthalmol. 2018;136(11):1242–8. 10.1001/jamaophthalmol.2018.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Querques G, et al. Functional and morphological changes of the retinal vessels in Alzheimer’s disease and mild cognitive impairment. Sci Rep. 2019;9(1):63. 10.1038/s41598-018-37271-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon SP, et al. Retinal microvascular and neurodegenerative changes in Alzheimer’s disease and mild cognitive impairment compared with control participants. Ophthalmol Retina. 2019;3(6):489–99. 10.1016/j.oret.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang YS, et al. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer’s disease on optical coherence tomography angiography. PLoS ONE. 2019;14(4):e0214685. 10.1371/journal.pone.0214685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Kreeke JA, et al. Optical coherence tomography angiography in preclinical Alzheimer’s disease. Br J Ophthalmol. 2020;104(2):157–61. 10.1136/bjophthalmol-2019-314127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CS, Apte RS. Retinal biomarkers of Alzheimer disease. Am J Ophthalmol. 2020;218:337–41. 10.1016/j.ajo.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashraf M, et al. Optical coherence tomography angiography projection artifact removal: impact on capillary density and interaction with diabetic retinopathy severity. Transl Vis Sci Technol. 2020;9(7):10. 10.1167/tvst.9.7.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Cuenca I, Salobrar-García E, Elvira-Hurtado L, Fernández-Albarral JA, Sánchez-Puebla L, Salazar JJ, Ramírez JM, Ramírez AI, de Hoz R. The Value of OCT and OCTA as Potential Biomarkers for Preclinical Alzheimer’s Disease: A Review Study. Life (Basel, Switzerland). 2021;11(7):712. 10.3390/life11070712. 10.3390/life11070712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inada K, et al. Increase of aqueous humor proteins with aging. Jpn J Ophthalmol. 1988;32(2):126–31. [PubMed] [Google Scholar]
- 42.Tripathi RC, et al. Tau fraction of transferrin is present in human aqueous humor and is not unique to cerebrospinal fluid. Exp Eye Res. 1990;50(5):541–7. 10.1016/0014-4835(90)90043-T [DOI] [PubMed] [Google Scholar]
- 43.Yoneda S, et al. Vitreous fluid levels of beta-amyloid((1–42)) and tau in patients with retinal diseases. Jpn J Ophthalmol. 2005;49(2):106–8. 10.1007/s10384-004-0156-x [DOI] [PubMed] [Google Scholar]
- 44.Lim JK, et al. The eye as a biomarker for Alzheimer’s disease. Front Neurosci. 2016;10:536. 10.3389/fnins.2016.00536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright LM, et al. Association of cognitive function with amyloid-beta and tau proteins in the vitreous humor. J Alzheimers Dis. 2019;68(4):1429–38. 10.3233/JAD-181104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein LE, et al. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet. 2003;361(9365):1258–65. 10.1016/S0140-6736(03)12981-9 [DOI] [PubMed] [Google Scholar]
- 47.Chiu K, et al. Neurodegeneration of the retina in mouse models of Alzheimer’s disease: what can we learn from the retina? Age (Dordr). 2012;34(3):633–49. 10.1007/s11357-011-9260-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez SE, et al. Beta-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2009;50(2):793–800. 10.1167/iovs.08-2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu B, et al. Amyloid-peptide vaccinations reduce beta-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer’s transgenic mice. Am J Pathol. 2009;175(5):2099–110. 10.2353/ajpath.2009.090159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ning A, et al. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci. 2008;49(11):5136–43. 10.1167/iovs.08-1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai Y, et al. Ocular changes in TgF344-AD rat model of Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2014;55(1):523–34. 10.1167/iovs.13-12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang J, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733–6. 10.1038/325733a0 [DOI] [PubMed] [Google Scholar]
- 53.Koronyo-Hamaoui M, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54(Suppl 1):S204–17. 10.1016/j.neuroimage.2010.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerbage C, et al. Detection of amyloid beta signature in the lens and its correlation in the brain to aid in the diagnosis of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2015;30(8):738–45. 10.1177/1533317513520214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.La Morgia C, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 2016;79(1):90–109. 10.1002/ana.24548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barmada A, Shippy SA. Tear analysis as the next routine body fluid test. Eye (Lond). 2020;34(10):1731–3. 10.1038/s41433-020-0930-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Posa A, et al. Schirmer strip vs. capillary tube method: non-invasive methods of obtaining proteins from tear fluid. Ann Anat. 2013;195(2):137–42. 10.1016/j.aanat.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 58.Xiao Z, et al. Plasma biomarker profiles and the correlation with cognitive function across the clinical spectrum of Alzheimer’s disease. Alzheimers Res Ther. 2021;13(1):123. 10.1186/s13195-021-00864-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smirnov DS, et al. Plasma biomarkers for Alzheimer’s Disease in relation to neuropathology and cognitive change. Acta Neuropathol. 2022;143(4):487–503. 10.1007/s00401-022-02408-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Data is provided within the manuscript. Moreover, the datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.