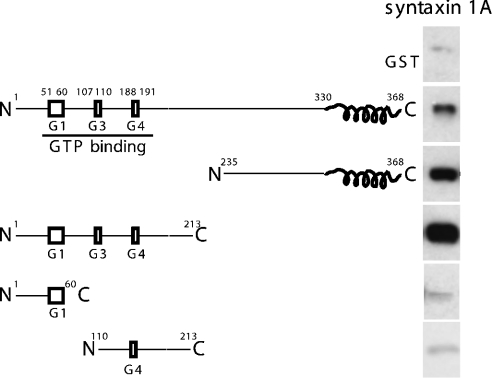
Figure 1. Specific interaction of truncation mutants of Sept5 with syntaxin in vitro.
GST alone or truncation mutants of Sept5 (0.1 nmol) were bound to glutathione–agarose and incubated with soluble recombinant syntaxin 1A (0.2 nmol). After washing, syntaxin bound to the various constructs was analysed by SDS/PAGE and immunoblotting with anti-syntaxin antibodies. Whereas GST alone or GST–Sept5 truncations of residues 1–60 and 110–213 did not appreciably bind syntaxin, the binding of the N-terminal region of Sept5 to syntaxin is stronger than that of full-length or C-terminal region of Sept5.