Figure 6.
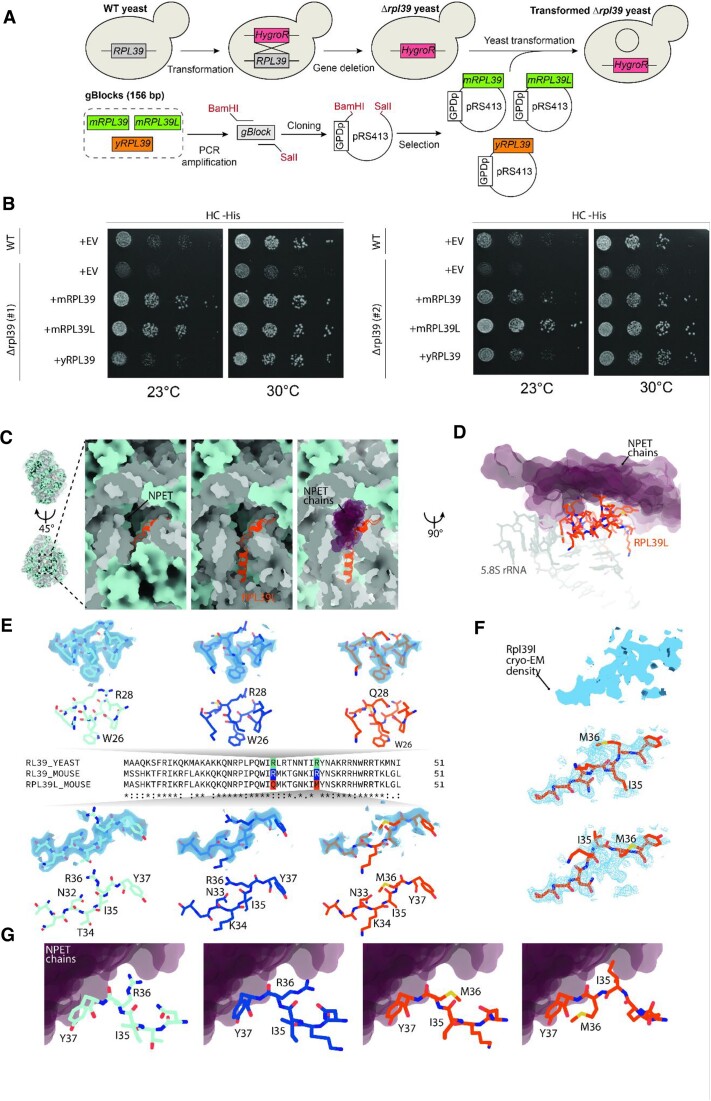
RPL39L introduces a hydrophobic patch in the NPET. (A) Scheme of yeast RPL39 KO and insertion of mouse RPL39 and mouse RPL39L. (B) The RPL39 KO causes growth defects in yeast upon environmental challenges like growth at 23 degrees Celsius in HC-His media. Expression of mouse RPL39 or RPL39L rescues these phenotypes. +EV are cells transformed with an empty vector as control. The experiments were carried out in two independent RPL39 knockout clones (#1 and #2). (C) RPL39L (cartoon representation, orange) shown in context of the large ribosomal subunit (refined atomic model of rpl39Δ-MmRpl39l; rRNAs are shown in gray, proteins are shown in pale blue surface representation). RPL39L is embedded inside the 60S subunit, adjacent to the exit of the NPET. Part of the 5.8S rRNA and ribosomal proteins have been removed for clarity on the center and right panel. Nascent protein chains and regulatory protein complexes pass the NPET in direct proximity to RPL39L as evident from aligned structures containing NPET-bound chains (nascent chains and regulatory proteins, PDB-IDs: 6M62, 7OBR, 7TM3, 7TUT, 7QWQ, 7QWS, shown as purple semi-transparent surfaces). (D) Side view of RPL39L (orange) and the surrounding 5.8S rRNA (gray) in direct contact with the NPET-facing region of RPL39L. The region containing Q28 and M36 is located directly adjacent to protein chains localized in the lumen of the NPET in structures containing nascent chains or tunnel-bound regulatory complexes (protein chain models shown as purple semi-transparent surfaces). (E) Comparison of the atomic model in the immediate surrounding of R/Q28 and R/M36 in WT yeast RPL39 (cyan), mouse RPL39 (dark blue), and mouse RPL39L (orange), shown in stick representation. The experimental cryo-EM density (semi-transparent surface, light blue) is shown superimposed on the refined atomic model. Maps around R28 are shown at a threshold of 6.5σ (yRPL39), 5.5σ (mRPL39), and 4.25σ (mRPL39L), while maps around R/M36 at a threshold of 5σ (yRPL39), 4σ (mRPL39), and 3.75σ (mRPL39L). Experimental cryo-EM density around M36 in mRPL39L is substantially weaker than the density observed either in yeast or mouse RPL39, due to increased conformational heterogeneity. (F) At a lower threshold, an alternative conformation is apparent in the experimental cryo-EM density (blue surface) of the region around M36 in mRPL39L, as RPL39L adopts two alternative conformations that differ substantially relative to the protein backbone and side chains. (G) In both WT yRPL39 (atomic model, stick representation, cyan) and mRPL39 (dark blue), the side chain of R36 faces the lumen of the NPET, potentially in direct contact with the protein chains inside the NPET (fitted chains of nascent protein and regulatory complexes, shown as purple semi-transparent surfaces). In mRPL39L, side chains of M36 and I35 hydrophobic residues are facing the NPET chains, forming a hydrophobic spot inside the NPET.