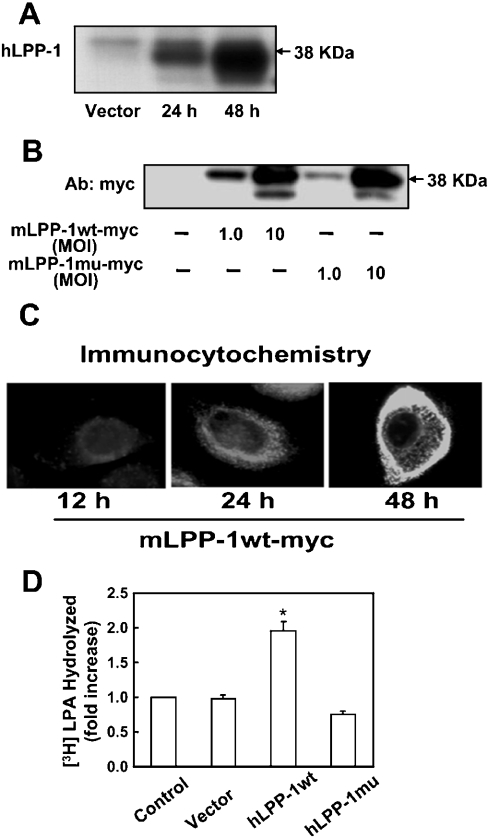
Figure 3. Overexpression of adenoviral constructs of LPP-1 wt and LPP-1 mutant in HBEpCs.
(A) HBEpCs (∼70% confluence in 35 mm dishes) were infected with empty vector or hLPP-1 wt adenoviral construct (MOI 25) in complete BEBM for 24 and 48 h. At the indicated time points, cell lysates were prepared as described in the Experimental section, and subjected to SDS/PAGE and Western blotting with anti-hLPP-1 antibody. (B) HBEpCs (∼70% confluence) were infected with empty vector, Myc-tagged mLPP-1 wt or Myc-tagged mLPP-1 mutant adenoviral constructs (MOI 1 and 10) in complete BEBM for 24 h. Cell lysates were subjected to SDS/PAGE and Western blotting with anti-Myc antibody (Ab). (C) HBEpCs grown on coverslips to ∼70% confluence were infected with Myc-tagged mLPP-1 wt in adenoviral constructs (MOI 10) for 12, 24 h and 48 h. Cells were subjected to immunostaining with anti-Myc antibody (9E10) and examined by fluorescent microscopy. Each immunofluorescence image is representative of three independent experiments. (D) HBEpCs (∼70% confluence) were infected with empty vector, hLPP-1 wt or hLPP-1 mutant adenoviral constructs (MOI 25) for 48 h. [3H]LPA (1 μM; specific radioactivity 2.2×103 d.p.m./pmol) complexed with 0.1% BSA in BEBM was added to each dish and dephosphorylation was examined at the end of a 30 min incubation. Lipids were extracted from the medium under acidic conditions and separated by TLC on silica gel H developed in hexane/diethyl ether/acetic acid (60:40:1, by vol.) as the solvent system. Unlabelled MOG was added as carrier, and radioactivity associated with the dephosphorylated product of LPA was quantified by counting in a scintillation spectrometer and corrected to d.p.m. Values are means±S.D. of three independent experiments, and are expressed as fold increase in LPA hydrolysis; *P<0.05 compared with vector control.