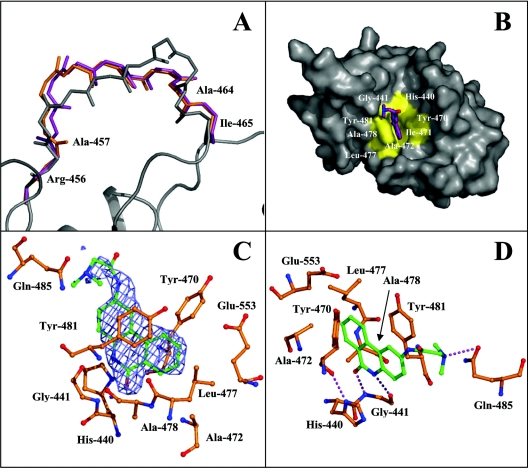
Figure 3. Structure of PE24H–PJ34.
(A) Superposition of the modelled loop from the catalytic domain of ETA (PE24H) in complex with PJ34 [monomer A (shown in grey)] compared with the loops from previous ETA structures. The loop from the catalytic domain of ETA in complex with either β-TAD (shown in purple, monomer B; PDB number 1AER [8]) or hydrolysed NAD+ (shown in orange, monomer B; PDB number 1DMA [12] are illustrated. The main chain is shown from residues 456 to 465. (B) The PJ34 inhibitor bound within the active site of the toxin. The hydrophobic pocket is shown in yellow and the inhibitor in purple. This hydrophobic pocket comprises the residues His-440, Gly-441, Tyr-470, Ile-471, Ala-472, Leu-477, Ala-478 and Tyr-481. (C) The 2Fobs-Fcalc omit map of PJ34 bound to monomer A within the active site of ETA contoured at 1σ. Phases for the map were calculated with no contribution from the PJ34 atoms. (D) The binding of the inhibitor PJ34 to PE24H. PJ34 is shown in green and important toxin residues involved in hydrogen bonding (broken lines) or hydrophobic interactions are shown.