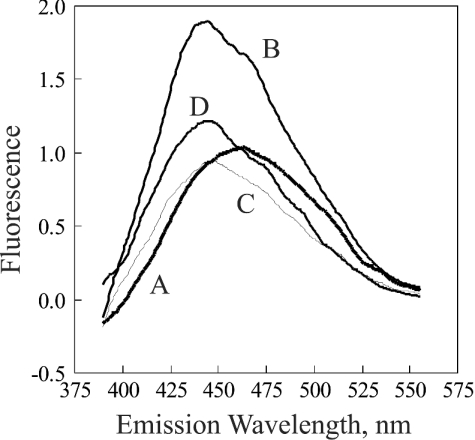
Figure 5. Fluorescence spectra of catalase exposed to H2O2.
The excitation wavelength was 340 nm. The catalase was mixed with NADPH in the ratio of 1.0 nmol of NADPH per nmol of catalase. Excess NADPH was removed by a single ultrafiltration–wash (see the Experimental section). The spectra are of the following in 0.05 M phosphate buffer, pH 6.5, after subtraction of the spectrum of the phosphate buffer: A, 1.0 μM NADPH; B, 1.0 μM bovine liver catalase without exposure to H2O2; C, 0.95 μM catalase after 1 h of exposure to H2O2 generated by glucose and glucose oxidase; D, 0.95 μM catalase after 1 h of exposure (at a catalase concentration of 7.6 μM) to H2O2. For both C and D, the rate of generation of H2O2 was 60 nmol·h−1 per nmol of catalase at 37 °C.