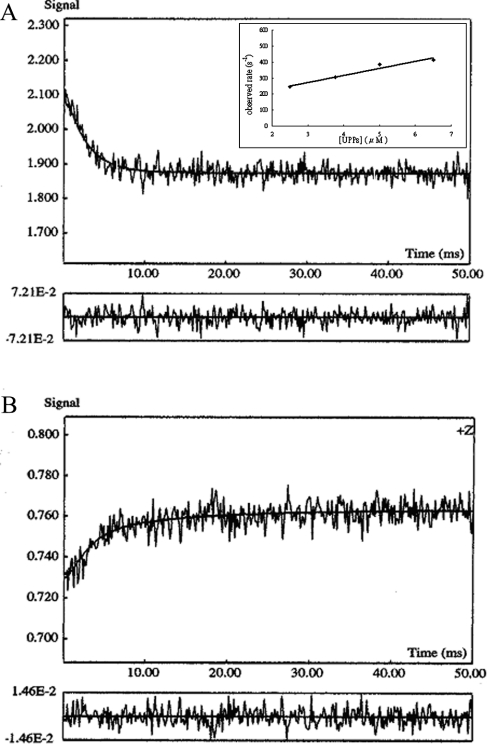
Figure 3. UPPS binding assay by stopped-flow spectrofluorimetry.
(A) Binding process of UPPS with TFMC-GP was monitored by using the stopped-flow spectrofluorimeter. A representative stopped-trace obtained by mixing 0.5 μM TFMC-GP with an equal volume of 5 μM UPPS is shown. The data were fitted with a single exponential equation to obtain kobs=384±21 s−1. The kon was determined from the slope of the plot of kobs values versus concentrations of the compound shown in the inset of this Figure. (B) The increase in fluorescence by mixing an equal volume of 0.5 μM enzyme preincubated with 7.5 μM TFMC-GP with 25 μM excessive FPP. By fitting the data with a single exponential equation, the rate constant for the dissociation of the compound from UPPS was determined to be 192±14.7 s−1.