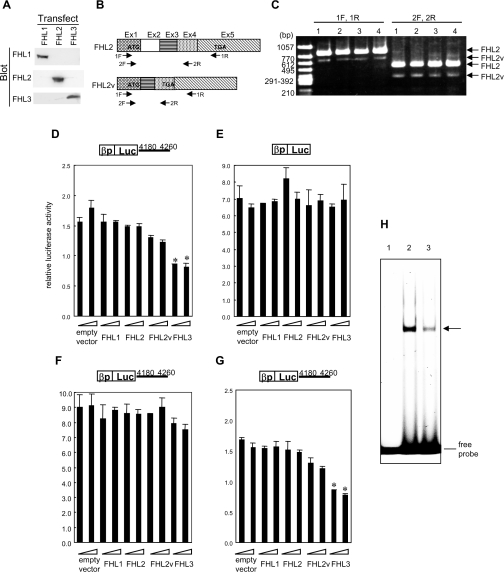
Figure 5. FHL3 down-regulated the FcεRI β-chain gene promoter activity through the element in the fourth intron in an MZF-1-dependent manner.
(A) Expression of FHL1, FHL2 and FHL3 in KU812 cells transfected with each expression plasmid was detected by Western blotting with anti-FHL1, anti-FHL2 and anti-FHL3 antibodies. (B) cDNA structures of full-length FHL2 (FHL2) and a splice variant (FHL2v) are schematically represented. (C) Expression of mRNAs for FHL2 and FHL2v in various cell lines was detected by RT–PCR employing two primer sets (1F/1R and 2F/2R). Lane 1, KU812 cells; lane 2, HMC-1 cells; lane 3, Jurkat cells; lane 4, HeLa cells. The primers employed for PCR are indicated in (B). (D–G) For a transient expression assay, expression plasmids of FHL3 and other members of the FHL family (FHL1, FHL2 and FHL2v) were introduced into KU812 cells with a reporter plasmid carrying a luciferase gene under the control of the human FcεRI β-chain promoter in the presence (D, F, G) or absence (E) of the β-chain gene region 4180–4260 nt, which mediates the transcriptional repression by MZF-1. In addition, MZF-1 antisense (F) or the same amount of a scrambled oligonucleotide as a control (G) was introduced into the cells. Luciferase activities relative to that from a reporter construct solely containing a luciferase gene without a promoter are shown. Results are expressed as the means±S.D. for three independent experiments. *P<0.05, significantly different from the cells transfected with an equivalent amount of an empty vector. (H) A double-stranded oligonucleotide probe carrying a consensus sequence of MZF-1-binding motif and the nuclear extracts from the cells transfected with MZF-1 antisense or an equivalent amount of a scrambled oligonucleotide were employed for EMSA. Lane 1, no nuclear extract; lane 2, nuclear extracts of the cells in which a scrambled oligonucleotide was introduced; lane 3, nuclear extracts of MZF-1 antisense-introduced cells.