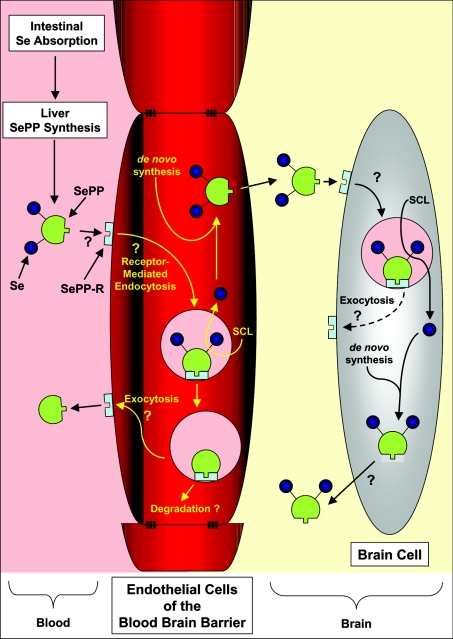
Figure 1. Hypothetical model of Se transport via SePP and its uptake mechanism by the brain and other tissues.
Dietary-derived Se is taken up by the liver, and is used for the synthesis of SePP. The release of SePP into the plasma provides Se that is used by distant tissues. SePP may donate Se to cells via its binding to a postulated SePP receptor (SePP-R) [3,9]. Such a delivery mechanism is necessary for targeting Se to tissues [4]. This is not unreasonable to suggest, as it is pre-empted by the mechanism by which Tf donates Fe to cells and also transfers Fe across the blood–brain barrier [10]. Delivery of Se from SePP to the brain may involve uptake of the protein via the SePP-R on the blood–brain barrier endothelial cells, its subsequent internalization and the release of Se, perhaps via the recently identified selenocysteine lyase (SCL) [6]. Whether, after Se release, the SePP and SePP-R can be recycled to the cell surface for re-utilization, like Tf and its receptor [10], or is degraded remains unknown, but testable. Se may then be re-incorporated into de novo synthesized SePP that is then released from the abluminal membrane of the endothelial cell into the interstitial space of the brain. Uptake of SePP via SePP-R on brain cells and the release of Se via SCL could provide the cell with Se for metabolism. De novo synthesis of brain SePP would result in local ‘organification of Se’, while release of some of this newly synthesized SePP would provide an extracellular ‘store of Se’ that can be utilized by brain cells [6]. This mechanism would explain why the brain does not lose Se during times of Se restriction, as it is trapped within the ‘selenoprotein cycle’ [6].